Yonsei Med J.
2018 Mar;59(2):202-210. 10.3349/ymj.2018.59.2.202.
Efficacy of Pemetrexed-based Chemotherapy in Comparison to Non-Pemetrexed-based Chemotherapy in Advanced, ALK+ Non-Small Cell Lung Cancer
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea. jslee@snubh.org
- 2Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea.
- 3Department of Pathology, Seoul National University Bundang Hospital, Seongnam, Korea.
- 4Department of Pathology, Seoul National University Hospital, Seoul, Korea.
- KMID: 2418782
- DOI: http://doi.org/10.3349/ymj.2018.59.2.202
Abstract
- PURPOSE
Previous retrospective studies suggest that anaplastic lymphoma kinase (ALK) mutation-positive (ALK+) non-small cell lung cancer (NSCLC) patients are sensitive to pemetrexed. To determine its efficacy, we retrospectively evaluated clinical outcomes of pemetrexed-based chemotherapy in patients with ALK+ NSCLC.
MATERIALS AND METHODS
We identified 126 patients with advanced, ALK+ NSCLC who received first-line cytotoxic chemotherapy. We compared response, progression-free survival (PFS), and overall survival (OS) rates according to chemotherapy regimens. Furthermore, we evaluated intracranial time to tumor progression (TTP) and proportion of ALK+ cells as prognostic factors.
RESULTS
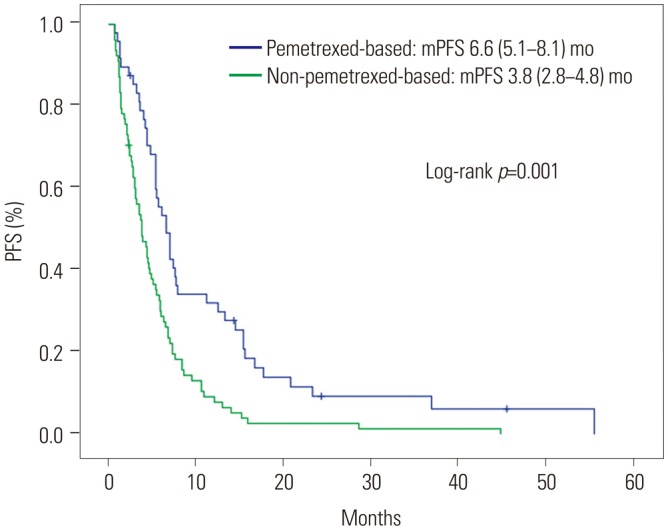
Forty-eight patients received pemetrexed-based chemotherapy, while 78 received other regimens as first-line treatment. The pemetrexed-based chemotherapy group showed superior overall response (44.7% vs. 14.3%, p < 0.001) and disease control (85.1% vs. 62.3%, p=0.008) rates. The pemetrexed-based chemotherapy group also exhibited longer PFS (6.6 months vs. 3.8 months, p < 0.001); OS rates were not significantly different. The lack of exposure to second-generation ALK inhibitors and intracranial metastasis on initial diagnosis were independent negative prognostic factors of OS. Intracranial TTP was similar between the treatment groups (32.7 months vs. 35.7 months, p=0.733). Patients who harbored a greater number of ALK+ tumor cells (≥70%) showed prolonged OS on univariate analysis (not reached vs. 44.8 months, p=0.041), but not on multivariate analysis (hazard ratio: 0.19, 95% confidence interval: 0.03-1.42; p=0.106).
CONCLUSION
Pemetrexed-based regimens may prolong PFS in patients with ALK+ NSCLC as a first-line treatment, but are not associated with prolonged OS. Exposure to second-generation ALK inhibitors may improve OS rates in patients with ALK+ NSCLC.
MeSH Terms
-
Adult
Aged
Antineoplastic Agents/*therapeutic use
Carcinoma, Non-Small-Cell Lung/*drug therapy/enzymology/mortality
Disease-Free Survival
Female
Humans
Lung Neoplasms/*drug therapy/enzymology/mortality
Male
Middle Aged
Mutation
Pemetrexed/*therapeutic use
Receptor Protein-Tyrosine Kinases/genetics
Retrospective Studies
Survival Rate
Treatment Outcome
Antineoplastic Agents
Pemetrexed
Receptor Protein-Tyrosine Kinases
Figure
Reference
-
1. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012; 13:239–246. PMID: 22285168.2. Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. PROFILE 1014 Investigators. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014; 371:2167–2177. PMID: 25470694.
Article3. Hyman DM, Puzanov I, Subbiah V, Faris JE, Chau I, Blay JY, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015; 373:726–736. PMID: 26287849.
Article4. Shaw AT, Ou SH, Bang YJ, Camidge DR, Solomon BJ, Salgia R, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014; 371:1963–1971. PMID: 25264305.
Article5. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015; 373:1627–1639. PMID: 26412456.
Article6. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015; 373:123–135. PMID: 26028407.
Article7. Kim DW, Mehra R, Tan DS, Felip E, Chow LQ, Camidge DR, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 2016; 17:452–463. PMID: 26973324.
Article8. Ou SH, Ahn JS, De Petris L, Govindan R, Yang JC, Hughes B, et al. Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: a phase II global study. J Clin Oncol. 2016; 34:661–668. PMID: 26598747.
Article9. Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008; 26:3543–3551. PMID: 18506025.
Article10. Paz-Ares L, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012; 13:247–255. PMID: 22341744.
Article11. Park S, Park TS, Choi CM, Lee DH, Kim SW, Lee JS, et al. Survival benefit of pemetrexed in lung adenocarcinoma patients with anaplastic lymphoma kinase gene rearrangements. Clin Lung Cancer. 2015; 16:e83–e89. PMID: 25682546.
Article12. Camidge DR, Kono SA, Lu X, Okuyama S, Barón AE, Oton AB, et al. Anaplastic lymphoma kinase gene rearrangements in non-small cell lung cancer are associated with prolonged progression-free survival on pemetrexed. J Thorac Oncol. 2011; 6:774–780. PMID: 21336183.
Article13. Lee JO, Kim TM, Lee SH, Kim DW, Kim S, Jeon YK, et al. Anaplastic lymphoma kinase translocation: a predictive biomarker of pemetrexed in patients with non-small cell lung cancer. J Thorac Oncol. 2011; 6:1474–1480. PMID: 21642865.
Article14. Shaw AT, Varghese AM, Solomon BJ, Costa DB, Novello S, Mino-Kenudson M, et al. Pemetrexed-based chemotherapy in patients with advanced, ALK-positive non-small cell lung cancer. Ann Oncol. 2013; 24:59–66. PMID: 22887466.
Article15. Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010; 363:1693–1703. PMID: 20979469.16. Schwartz LH, Bogaerts J, Ford R, Shankar L, Therasse P, Gwyther S, et al. Evaluation of lymph nodes with RECIST 1.1. Eur J Cancer. 2009; 45:261–267. PMID: 19091550.
Article17. Sun JM, Ahn JS, Jung SH, Sun J, Ha SY, Han J, et al. Pemetrexed plus cisplatin versus gemcitabine plus cisplatin according to thymidylate synthase expression in nonsquamous non-small-cell lung cancer: a biomarker-stratified randomized phase II trial. J Clin Oncol. 2015; 33:2450–2456. PMID: 26124486.
Article18. Xu CW, Wang G, Wang WL, Gao WB, Han CJ, Gao JS, et al. Association between EML4-ALK fusion gene and thymidylate synthase mRNA expression in non-small cell lung cancer tissues. Exp Ther Med. 2015; 9:2151–2154. PMID: 26136951.
Article19. Takezawa K, Okamoto I, Okamoto W, Takeda M, Sakai K, Tsukioka S, et al. Thymidylate synthase as a determinant of pemetrexed sensitivity in non-small cell lung cancer. Br J Cancer. 2011; 104:1594–1601. PMID: 21487406.
Article20. Berge EM, Lu X, Maxson D, Barón AE, Gadgeel SM, Solomon BJ, et al. Clinical benefit from pemetrexed before and after crizotinib exposure and from crizotinib before and after pemetrexed exposure in patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer. Clin Lung Cancer. 2013; 14:636–643. PMID: 23931899.
Article21. Rangachari D, Yamaguchi N, VanderLaan PA, Folch E, Mahadevan A, Floyd SR, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer. 2015; 88:108–111. PMID: 25682925.22. Costa DB, Shaw AT, Ou SH, Solomon BJ, Riely GJ, Ahn MJ, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. 2015; 33:1881–1888. PMID: 25624436.
Article23. Costa DB, Kobayashi S, Pandya SS, Yeo WL, Shen Z, Tan W, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011; 29:e443–e445. PMID: 21422405.
Article24. Gettinger SN, Bazhenova LA, Langer CJ, Salgia R, Gold KA, Rosell R, et al. Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: a single-arm, open-label, phase 1/2 trial. Lancet Oncol. 2016; 17:1683–1696. PMID: 27836716.25. Gandhi L, Drappatz J, Ramaiya NH, Otterson GA. High-dose pemetrexed in combination with high-dose crizotinib for the treatment of refractory CNS metastases in ALK-rearranged non-small-cell lung cancer. J Thorac Oncol. 2013; 8:e3–e5. PMID: 23242445.
Article26. Moro-Sibilot D, Smit E, de Castro, Lesniewski-Kmak K, Aerts JG, Villatoro R, et al. Non-small cell lung cancer patients with brain metastases treated with first-line platinum-doublet chemotherapy: analysis from the European FRAME study. Lung Cancer. 2015; 90:427–432. PMID: 26791802.
Article27. Zhu W, Røe OD, Wu C, Li W, Guo R, Gu Y, et al. Activity of pemetrexed-based regimen as first-line chemotherapy for advanced non-small cell lung cancer with asymptomatic inoperable brain metastasis: a retrospective study. J Chemother. 2015; 27:221–226. PMID: 25735792.
Article28. Tanaka T, Yoshioka H, Haratani K, Hayashi H, Okamoto K, Kaneda T, et al. The association between the percentage of anaplastic lymphoma kinase(ALK)-positive cells and efficacy of ALK inhibitor (P3.02a-005). J Thorac Oncol. 2017; 12:S1162.29. Lei YY, Yang JJ, Zhang XC, Zhong WZ, Zhou Q, Tu HY, et al. Anaplastic lymphoma kinase variants and the percentage of ALK-positive tumor cells and the efficacy of crizotinib in advanced NSCLC. Clin Lung Cancer. 2016; 17:223–231. PMID: 26454342.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Interstitial Pneumonitis after Treatment with Pemetrexed for Non-small Cell Lung Cancer
- Efficacy and Safety of Pemetrexed in Advanced Non-Small Cell Lung Carcinoma
- Pemetrexed Continuation Maintenance versus Conventional Platinum-Based Doublet Chemotherapy in EGFR-Negative Lung Adenocarcinoma: Retrospective Analysis
- Epiphora following chemotherapy with pemetrexed in patients with advanced non-small cell lung cancer
- Chemotherapy in Advanced Urothelial Carcinoma