Cancer Res Treat.
2013 Mar;45(1):74-77.
Interstitial Pneumonitis after Treatment with Pemetrexed for Non-small Cell Lung Cancer
- Affiliations
-
- 1Department of Internal Medicine, Kangwon National University Hospital, Kangwon National University School of Medicine, Chuncheon, Korea. hylee7@kangwon.ac.kr
- 2Department of Emergency Medicine, Kangwon National University Hospital, Kangwon National University School of Medicine, Chuncheon, Korea.
- 3Department of Pathology, Kangwon National University Hospital, Kangwon National University School of Medicine, Chuncheon, Korea.
Abstract
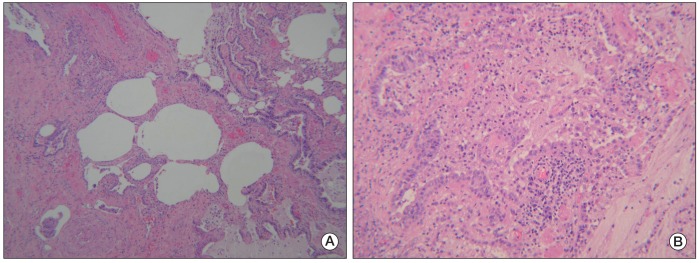
- Pemetrexed is approved as a first-line treatment for advanced non-squamous non-small cell lung cancer (NSCLC) with cisplatin and as a single agent for second-line treatment or for patients who show no disease progression after four cycles of platinum-based doublet induction chemotherapy as maintenance therapy. Pemetrexed has a modest toxicity profile and has not traditionally been regarded as a cause of interstitial pneumonitis. Here, we report on a rare case of pemetrexed-induced pneumonitis in a patient with NSCLC.
Keyword
MeSH Terms
Figure
Reference
-
1. Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008; 26:3543–3551. PMID: 18506025.
Article2. Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004; 22:1589–1597. PMID: 15117980.
Article3. Ciuleanu T, Brodowicz T, Zielinski C, Kim JH, Krzakowski M, Laack E, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009; 374:1432–1440. PMID: 19767093.
Article4. Sun JM, Lee KW, Kim JH, Kim YJ, Yoon HI, Lee JH, et al. Efficacy and toxicity of pemetrexed as a third-line treatment for non-small cell lung cancer. Jpn J Clin Oncol. 2009; 39:27–32. PMID: 18952704.
Article5. Ohe Y, Ichinose Y, Nakagawa K, Tamura T, Kubota K, Yamamoto N, et al. Efficacy and safety of two doses of pemetrexed supplemented with folic acid and vitamin B12 in previously treated patients with non-small cell lung cancer. Clin Cancer Res. 2008; 14:4206–4212. PMID: 18594001.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Epiphora following chemotherapy with pemetrexed in patients with advanced non-small cell lung cancer
- A Case of Nonspecific Interstitial Pneumonitis Improved After Cyclosporin Therapy
- Factors predicting radiation pneumonitis in locally advanced non-small cell lung cancer
- A Good Outcome for a Case of Chronic Pneumonitis of Infancy
- Efficacy and Safety of Pemetrexed in Advanced Non-Small Cell Lung Carcinoma