Korean J Radiol.
2018 Oct;19(5):950-956. 10.3348/kjr.2018.19.5.950.
Effect of Poly(Lactide-Co-Glycolide) Nanoparticles on Local Retention of Fluorescent Material: An Experimental Study in Mice
- Affiliations
-
- 1Department of Radiology, Seoul Metropolitan Government-Seoul National University Boramae Hospital, Seoul 07061, Korea.
- 2Department of Radiology, Seoul National University Bundang Hospital, Seongnam 13620, Korea. eugene801027@gmail.com
- 3College of Pharmacy, Chung-Ang University, Seoul 06911, Korea.
- 4College of Pharmacy, Dankook University, Cheonan 31116, Korea.
- KMID: 2418559
- DOI: http://doi.org/10.3348/kjr.2018.19.5.950
Abstract
OBJECTIVE
Poly(lactide-co-glycolide) (PLGA) nanoparticles are promising materials for the development of new drug-releasing systems. The purpose of this study was to evaluate the in vivo retention time of materials loaded in nanoparticles as compared with that of the material alone by in vivo imaging in nude mice.
MATERIALS AND METHODS
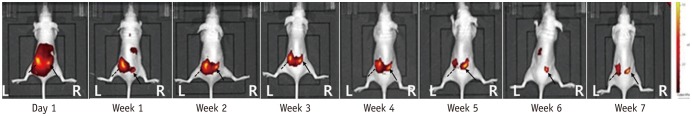
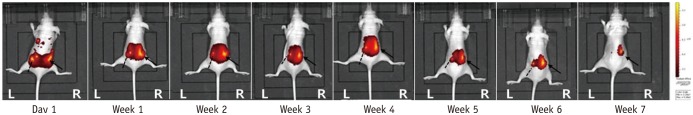
Mice (n = 20) were injected with 0.1 mL fluorescent material 1,1"²-dioctadecyl-3,3,3"²,3"² tetramethylindotricarbocyanine iodide (DiR)-loaded PLGA nanoparticles (200 nm) into the right paraspinal muscle, and the same volume of pure DiR solution was injected into the left paraspinal muscle. Fluorescence images were obtained using an in vivo optical imaging system. Fluorescent images were taken 1 day after the injection, and seven more images were taken at 1-week intervals. Image analysis was done with ImageJ program, and one region of interest was chosen manually, which corresponded to the highest signal-intensity area of fluorescence signal intensity.
RESULTS
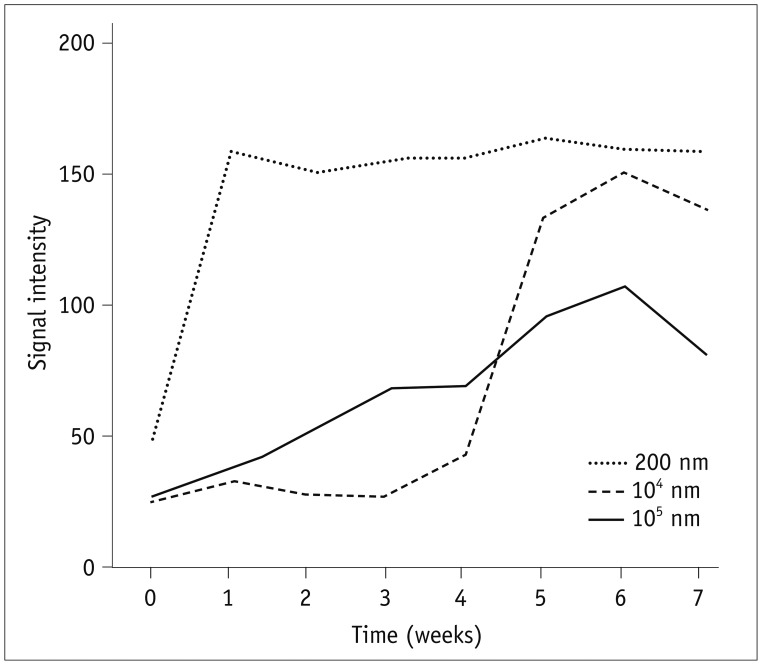
After 7 weeks, 12 mice showed a right-sided dominant signal, representing the DiR loaded PLGA nanoparticles; 5 mice showed a left-side dominant signal, representing the free DiR solution; and 3 mice showed no signal at all beginning 1 day after the injection. During the 7-week period, the mean signal intensities of the free DiR solution and DiR-loaded PLGA nanoparticles diverged gradually. On day 1, the mean signal intensity of free DiR solution was significantly higher than that of DiR-loaded PLGA (p < 0.001). Finally, by week 7, DiR-loaded PLGA express significantly high signal intensity compared with free DiR solution (p = 0.031).
CONCLUSION
The results of the current study suggested that therapeutic agents bound to PLGA nanoparticles may exhibit prolonged retention times.
MeSH Terms
Figure
Reference
-
1. Manchikanti L, Singh V, Pampati V, Beyer CD, Damron KS. Evaluation of the prevalence of facet joint pain in chronic thoracic pain. Pain Physician. 2002; 5:354–359. PMID: 16886012.
Article2. Fritz J, Niemeyer T, Clasen S, Wiskirchen J, Tepe G, Kastler B, et al. Management of chronic low back pain: rationales, principles, and targets of imaging-guided spinal injections. Radiographics. 2007; 27:1751–1771. PMID: 18025516.
Article3. Boswell MV, Trescot AM, Datta S, Schultz DM, Hansen HC, Abdi S, et al. Interventional techniques: evidence-based practice guidelines in the management of chronic spinal pain. Pain Physician. 2007; 10:7–111. PMID: 17256025.4. Fukui S, Ohseto K, Shiotani M, Ohno K, Karasawa H, Naganuma Y, et al. Referred pain distribution of the cervical zygapophyseal joints and cervical dorsal rami. Pain. 1996; 68:79–83. PMID: 9252002.
Article5. Fukui S, Ohseto K, Shiotani M, Ohno K, Karasawa H, Naganuma Y. Distribution of referred pain from the lumbar zygapophyseal joints and dorsal rami. Clin J Pain. 1997; 13:303–307. PMID: 9430810.
Article6. Boswell MV, Colson JD, Spillane WF. Therapeutic facet joint interventions in chronic spinal pain: a systematic review of effectiveness and complications. Pain Physician. 2005; 8:101–114. PMID: 16850048.7. Boswell MV, Colson JD, Sehgal N, Dunbar EE, Epter R. A systematic review of therapeutic facet joint interventions in chronic spinal pain. Pain Physician. 2007; 10:229–253. PMID: 17256032.8. Jarvik JG, Deyo RA. Diagnostic evaluation of low back pain with emphasis on imaging. Ann Intern Med. 2002; 137:586–597. PMID: 12353946.
Article9. Bogduk N. International Spinal Injection Society guidelines for the performance of spinal injection procedures. Part 1: zygapophysial joint blocks. Clin J Pain. 1997; 13:285–302. PMID: 9430809.10. Manchikanti L. Role of neuraxial steroids in interventional pain management. Pain Physician. 2002; 5:182–199. PMID: 16902669.
Article11. Kim YT, Caldwell JM, Bellamkonda RV. Nanoparticle-mediated local delivery of Methylprednisolone after spinal cord injury. Biomaterials. 2009; 30:2582–2590. PMID: 19185913.
Article12. Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008; 5:505–515. PMID: 18672949.
Article13. Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces. 2010; 75:1–18. PMID: 19782542.
Article14. Morgen M, Tung D, Boras B, Miller W, Malfait AM, Tortorella M. Nanoparticles for improved local retention after intra-articular injection into the knee joint. Pharm Res. 2013; 30:257–268. PMID: 22996566.
Article15. Leclerc L, Klein JP, Forest V, Boudard D, Martini M, Pourchez J, et al. Testicular biodistribution of silica-gold nanoparticles after intramuscular injection in mice. Biomed Microdevices. 2015; 17:66. PMID: 26044201.
Article16. Morishita Y, Yoshioka Y, Satoh H, Nojiri N, Nagano K, Abe Y, et al. Distribution and histologic effects of intravenously administered amorphous nanosilica particles in the testes of mice. Biochem Biophys Res Commun. 2012; 420:297–301. PMID: 22417826.
Article17. De Jong WH, Hagens WI, Krystek P, Burger MC, Sips AJ, Geertsma RE. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008; 29:1912–1919. PMID: 18242692.
Article18. Horisawa E, Kubota K, Tuboi I, Sato K, Yamamoto H, Takeuchi H, et al. Size-dependency of DL-lactide/glycolide copolymer particulates for intra-articular delivery system on phagocytosis in rat synovium. Pharm Res. 2002; 19:132–139. PMID: 11883639.19. Levick JR. A method for estimating macromolecular reflection by human synovium, using measurements of intra-articular half lives. Ann Rheum Dis. 1998; 57:339–344. PMID: 9771207.
Article20. Kim SR, Ho MJ, Lee E, Lee JW, Choi YW, Kang MJ. Cationic PLGA/Eudragit RL nanoparticles for increasing retention time in synovial cavity after intra-articular injection in knee joint. Int J Nanomedicine. 2015; 10:5263–5271. PMID: 26345227.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Encapsulation of Low Metronidazole Dose in Poly (D,L-lactide-co-glycolide) (PLGA) Nanoparticles Improves Giardia intestinalis Treatment
- Cellular responses on anodized titanium discs coated with 1 alpha,25-dihydroxyvitamin D3 incorporated Poly(D,L-lactide-co-glycolide) (PLGA) nanoparticles
- Induction of Potent Antigen-specific Cytotoxic T Cell Response by PLGA-nanoparticles Containing Antigen and TLR Agonist
- The sustaining effect of three polymers on the release of chlorhexidine from a controlled release drug device for root canal disinfection
- Effect of polyadenylic.polyuridylic acid on the proliferative responsiveness of mouse thymus and spleen cells