Immune Netw.
2013 Feb;13(1):30-33. 10.4110/in.2013.13.1.30.
Induction of Potent Antigen-specific Cytotoxic T Cell Response by PLGA-nanoparticles Containing Antigen and TLR Agonist
- Affiliations
-
- 1College of Pharmacy, Chungbuk National University, Cheongju 361-763, Korea. cklee@chungbuk.ac.kr
- KMID: 2150768
- DOI: http://doi.org/10.4110/in.2013.13.1.30
Abstract
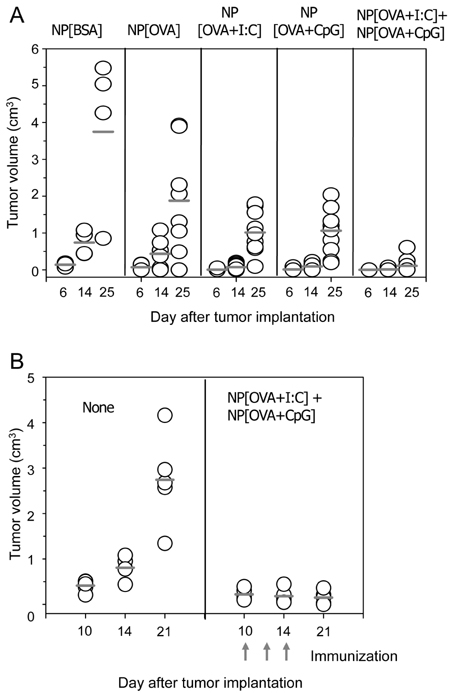
- Previously we showed that biodegradable nanoparticles containing poly-IC or CpG oligodeoxynucleotide (ODN) together with ovalbumin (OVA) were efficient at inducing MHC-restricted presentation of OVA peptides in dendritic cells. The CTL-inducing activities of the nanoparticles were examined in the present study. Nanoparticles containing poly-IC or CpG ODN together with OVA were prepared using biodegradable polymer poly(D,L-lactic acid-co-glycolic acid), and then were opsonized with mouse IgG. The nanoparticles were injected into the tail vein of mice, and 7 days later the OVA-specific CTL activities were measured using an in vivo CTL assay. Immunization of mice with the nanoparticles containing poly-IC or CpG ODN together with OVA elicited potent OVA-specific CTL activity compared to those containing OVA only. In accordance with these results, nanoparticles containing poly-IC or CpG ODN together with OVA exerted potent antitumor activity in mice that were subcutaneously implanted with EG7.OVA tumor cells. These results show that encapsulation of poly-IC or CpG ODN together with antigen in biodegradable nanoparticles is an effective approach for the induction of potent antigen-specific CTL responses in vivo.
Keyword
MeSH Terms
Figure
Reference
-
1. Van Der Bruggen P, Zhang Y, Chaux P, Stroobant V, Panichelli C, Schultz ES, Chapiro J, Van Den Eynde BJ, Brasseur F, Boon T. Tumor-specific shared antigenic peptides recognized by human T cells. Immunol Rev. 2002. 188:51–64.
Article2. Huang AY, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994. 264:961–965.
Article3. Harding CV. Phagocytic processing of antigens for presentation by MHC molecules. Trends Cell Biol. 1995. 5:105–109.
Article4. Sigal LJ, Crotty S, Andino R, Rock KL. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature. 1999. 398:77–80.
Article5. Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001. 19:47–64.
Article6. Carbone FR, Kurts C, Bennett SR, Miller JF, Heath WR. Cross-presentation: a general mechanism for CTL immunity and tolerance. Immunol Today. 1998. 19:368–373.
Article7. Shen H, Ackerman AL, Cody V, Giodini A, Hinson ER, Cresswell P, Edelson RL, Saltzman WM, Hanlon DJ. Enhanced and prolonged cross-presentation following endosomal escape of exogenous antigens encapsulated in biodegradable nanoparticles. Immunology. 2006. 117:78–88.
Article8. Gerelchuluun T, Lee YH, Lee YR, Im SA, Song S, Park JS, Han K, Kim K, Lee CK. Dendritic cells process antigens encapsulated in a biodegradable polymer, poly(D,L-lactide-co-glycolide), via an alternate class I MHC processing pathway. Arch Pharm Res. 2007. 30:1440–1446.
Article9. Lee YR, Lee YH, Im SA, Yang IH, Ahn GW, Kim K, Lee CK. Biodegradable nanoparticles containing TLR3 or TLR9 agonists together with antigen enhance MHC-restricted presentation of the antigen. Arch Pharm Res. 2010. 33:1859–1866.
Article10. Schliehe C, Redaelli C, Engelhardt S, Fehlings M, Mueller M, van Rooijen N, Thiry M, Hildner K, Weller H, Groettrup M. CD8-dendritic cells and macrophages cross-present poly(D,L-lactate-co-glycolate) acid microsphere-encapsulated antigen in vivo. J Immunol. 2011. 187:2112–2121.
Article11. Newman KD, Sosnowski DL, Kwon GS, Samuel J. Delivery of MUC1 mucin peptide by Poly (d,l-lactic-co-glycolic acid) microspheres induces type 1 T helper immune responses. J Pharm Sci. 1998. 87:1421–1427.
Article12. Venkataprasad N, Coombes AG, Singh M, Rohde M, Wilkinson K, Hudecz F, Davis SS, Vordermeier HM. Induction of cellular immunity to a mycobacterial antigen adsorbed on lamellar particles of lactide polymers. Vaccine. 1999. 17:1814–1819.
Article13. Lee YR, Yang IH, Lee YH, Im SA, Song S, Li H, Han K, Kim K, Eo SK, Lee CK. Cyclosporin A and tacrolimus, but not rapamycin, inhibit MHC-restricted antigen presentation pathways in dendritic cells. Blood. 2005. 105:3951–3955.
Article14. Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003. 55:329–347.
Article15. Moore MW, Carbone FR, Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988. 54:777–785.
Article16. Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003. 55:329–347.
Article17. Pfeifer JD, Wick MJ, Roberts RL, Findlay K, Normark SJ, Harding CV. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993. 361:359–362.
Article18. Elamanchili P, Diwan M, Cao M, Samuel J. Characterization of poly(D,L-lactic-co-glycolic acid) based nanoparticulate system for enhanced delivery of antigens to dendritic cells. Vaccine. 2004. 22:2406–2412.
Article19. Lee YH, Lee YR, Kim KH, Im SA, Song S, Lee MK, Kim Y, Hong JT, Kim K, Lee CK. Baccatin III, a synthetic precursor of taxol, enhances MHC-restricted antigen presentation in dendritic cells. Int Immunopharmacol. 2011. 11:985–991.
Article20. Lee YR, Lee YH, Im SA, Kim K, Lee CK. Formulation and characterization of antigen-loaded PLGA nanoparticles for efficient cross-priming of the antigen. Immune Netw. 2011. 11:163–168.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In vitro Stimulation of Tumor - Draining Lymph Node Lymphocytes with the 30 kDa Antigen of Mycobacterium tuberculosis Leads to the Differentiation of Th1 Cells and Cytotoxic Effector Cells
- Lumazine synthase protein cage nanoparticles as antigen delivery nanoplatforms for dendritic cell-based vaccine development
- Formulation and Characterization of Antigen-loaded PLGA Nanoparticles for Efficient Cross-priming of the Antigen
- Enhanced CEA-specific Immune Responses by Tat-LLO Fusion Protein
- In Vitro Induction of Carcinoembryonic Antigen (CEA) Specific Cytotoxic T Lymphocytes Using Dendritic Cells Pulsed with CEA Peptide