J Korean Acad Conserv Dent.
2004 Nov;29(6):548-554. 10.5395/JKACD.2004.29.6.548.
The sustaining effect of three polymers on the release of chlorhexidine from a controlled release drug device for root canal disinfection
- Affiliations
-
- 1Department of Conservative Dentistry, College of Dentistry, Yonsei University, Seoul, Korea. kum6139@yumc.yonsei.ac.kr
- 2Oral Science Research Center, Research Institute of Dental Biomaterials and Bioengineering, College of Dentistry, Yonsei University, Seoul, Korea.
- KMID: 2175656
- DOI: http://doi.org/10.5395/JKACD.2004.29.6.548
Abstract
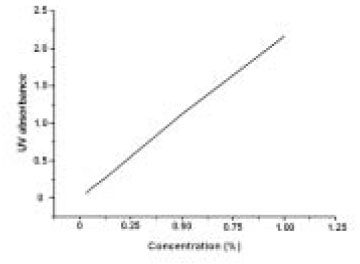
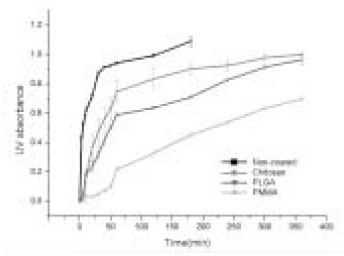
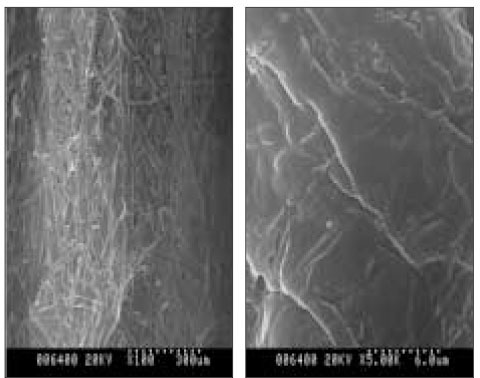
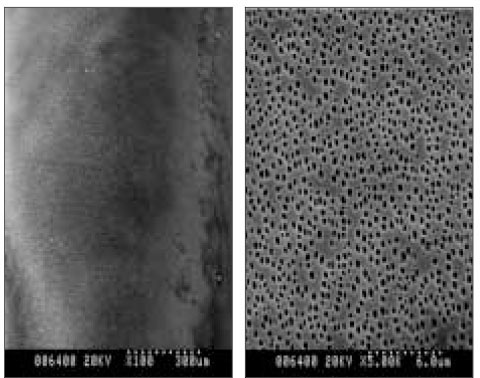
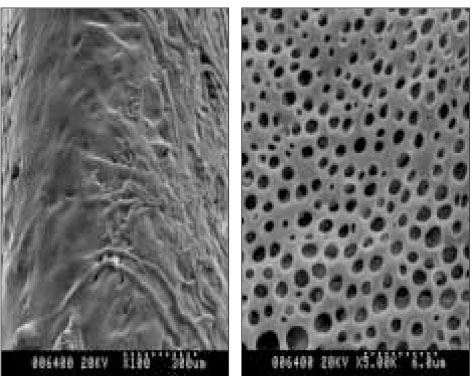
- The aim of this in vitro study was to evaluate the suitability of using chitosan, poly (lactide-co-glycolide) (PLGA), and polymethyl methacrylate (PMMA) to control the release of chlorhexidine digluconate (CHX) from a prototype of controlled release drug device (CRD) for root canal disinfection. Four different prototypes with different formulations were prepared. Group A (n = 12); The device (absorbent paper point) was loaded with CHX as control. Group B (n = 12); same as group A, but the device was coated with chitosan. In Groups C and D, the device was treated in the same way as group A and then coated three times with 5% PMMA (Group C, n = 12), or coated three times with 3% PLGA (Group D, n = 12). The devices were randomly allocated to experimental groups of 12 each. All CRD prototypes were soaked in 3 mL distilled water. The concentrations of CHX were determined using a UV spectrophotometer. The surface characteristics of each prototype were observed using a scanning electron microscope. The result showed that release rate of CHX was the greatest in the non-coated group, followed by the chitosan-coated group, the PLGA-coated group, and the PMMA-coated group (P < 0.05). Pores were observed on the surface of the prototypes that were coated with PLGA and PMMA. When the pore size was smaller, the release rate was lower. This data indicate that polymer coating can control the release rate of CHX from the CRD prototypes.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Effect of chlorhexidine application on the bond strength of resin core to axial dentin in endodontic cavity
Yun-Hee Kim, Dong-Hoon Shin
Restor Dent Endod. 2012;37(4):207-214. doi: 10.5395/rde.2012.37.4.207.Effect of different chlorhexidine application times on microtensile bond strength to dentin in Class I cavities
Hyun-Jung Kang, Ho-Jin Moon, Dong-Hoon Shin
Restor Dent Endod. 2012;37(1):9-15. doi: 10.5395/rde.2012.37.1.9.
Reference
-
1. Sjogren U, Figdor D, Persson S, Sundqvist G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endod J. 1997. 30:297–306.
Article2. Sundqvist G, Figdor D, Persson S, Sjogren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998. 85:86–93.
Article3. Friedman S, Komorowski R, Maillet W, Klimaite R, Nguyen HQ, Torneck CD. In vivo resistance of coronally induced bacterial ingress by an experimental glass ionomer cement root canal sealer. J Endod. 2000. 26:1–5.
Article4. Bystrom A, Claesson R, Sundqvist G. The antimicrobial effect of camphorated paramonochlorophenol, camphorated phenol and calcium hydroxide in the treatment of infected root canals. Endod Dent Traumatol. 1985. 1:170–175.
Article5. Cvek M, Hollender L, Nord CE. Treatment of non-vital permanent incisors with calcium hydroxide. Odontol Revy. 1976. 27:93–108.6. Orstavik D, Haapasalo M. Disinfection by endodontic irrigants and dressings by experimentally infected dentinal tubules. Endod Dent Traumatol. 1990. 6:142–149.
Article7. Haapasalo M, Ørstavik D. In-vitro infection and disinfection of dentinal tubules. J Dent Res. 1987. 66:1375–1379.8. Gomes BPFA, Souza SFC, Ferraz CCR, Teizeira FB, Zaia AA, Valdrigh L, Souza-Filho FJ. Effectiveness of 2% chlorhexidine gel and calcium hydroxide against Enterococcus faecalis in bovine root dentine in vitro. Int Endod J. 2003. 36:267–275.
Article9. Basrani B, Tajderhane L, Santos M, Pascon E, Grad H, Lawrence HP, Friedman S. Efficacy of chlorhexidine- and hydroxide-containing medicaments against Enterococcus faecalis in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003. 96:618–624.
Article10. Vianna ME, Gomes BP, Berber VB, Zaia AA, Ferraz CC, de Souza-Filho FJ. In vitro evaluation of the antimicrobial activity of chlorhexidine and sodium hypochlorite. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004. 97:79–84.
Article11. Jeansonne MJ, White RR. A comparison of 2% chlorhexidine gluconate and and 5.25% sodium hypochlorite as antimicrobial endodontic irrigants. J Endod. 1994. 20:276–278.
Article12. White RR, Hay GL, Janer LR. Residual antimicrobial activity after canal irrigation with chlorhexidine. J Endod. 1997. 23:229–231.
Article13. Basrani B, Santos JM, Tjaderhane L, Grad H, Gorduysus O, Huang J, Lawrence HP, Friedman S. Substantive antimicrobial activity in chlorhexidine-treated human root dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002. 94:240–245.
Article14. Komorowski R, Grad H, Wu Y, Friedman S. Antimicrobial substantivity of chlorhexedine-treated bovine root dentin. J Endod. 2000. 26:315–317.15. Jung S, Safavi K, Spangberg L. The effectiveness of Chlorhexidine in the prevention of root canal reinfection [abstract]. J Endod. 1999. 25:288.
Article16. Heling I, Sommer M, Steinberg D, Friedman M, Sela MN. Microbiological evaluation of the efficacy of chlorhexidine in a sustained-release device for dentine sterilization. Int Endod J. 1992. 25:15–19.
Article17. Heling I, Steinberg D, Kenig S, Gavrilovich I, Sela MN, Friedman M. Efficacy of a sustained-release device containing chlorhexidine and Ca (OH)2 in preventing secondary infection of dentinal tubules. Int Endod J. 1992. 25:20–24.
Article18. Miyazaki S, Yamaguchi H, Yokouchi C, Takada M, Hou WM. Sustained-release and intragastric-floating granules of indomethacin using chitosan in rabbits. Chem Pharm Bull. 1988. 36:4033–4038.
Article19. Anderson JM, Shive MS. Biodegradation and biocompatibility of PLA and PLGA microsphers. Adv Drug Deliv Rev. 1997. 28:5–24.20. Cam D, Hyon SH, Ikada Y. Degradation of high molecular weight poly (L-lactide) in alkaline medium. Biomaterials. 1995. 16:833–843.
Article21. Bayston R, Milner RDG. The sustained release of antimicrobial drugs from bone cement. J Bone Joint Surg Br. 1982. 64:460–464.22. Huang J, Wong HL, Zhou Y, Wu XY, Grad H, Komorowski R. In vitro studies and modeling of a controlled-release device for root canal therapy. J Control Release. 2000. 67:293–307.
Article23. Singla AK, Chawla M. Chitosan: some pharmaceutical and biological aspects-an update. J Pharm Pharmacol. 2001. 53:1047–1067.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Biodegradable poly (lactic acid) microspheres for drug delivery systems
- Reconsideration of treatment protocol on the reduction of Enterococcus faecalis associated with failed root canal treatment
- Evaluation of time-dependent antimicrobial effect of sodium dichloroisocyanurate (NaDCC) on Enterococcus faecalis in the root canal
- Preparation of Drug Eluting Natural Composite Scaffold Using Response Surface Methodology and Artificial Neural Network Approach
- Root canal irrigants influence the hydrophobicity and adherence of Staphylococcus epidermidis to root canal dentin: an in vitro study