Ann Rehabil Med.
2018 Jun;42(3):384-395. 10.5535/arm.2018.42.3.384.
Quantitative Evaluation of Post-stroke Spasticity Using Neurophysiological and Radiological Tools: A Pilot Study
- Affiliations
-
- 1Department of Rehabilitation Medicine, Konyang University College of Medicine, Daejeon, Korea. jbocean@hanmail.net
- 2Department of Radiology, Konyang University College of Medicine, Daejeon, Korea.
- 3Center for Medical Metrology, Korea Research Institute of Standards and Science (KRISS), Daejeon, Korea.
- 4University of Science and Technology, Daejeon, Korea.
- KMID: 2417830
- DOI: http://doi.org/10.5535/arm.2018.42.3.384
Abstract
OBJECTIVE
To determine the possibility of a new measurement tool using electromyography and ultrasonography for quantitative spasticity assessment in post-stroke patients.
METHODS
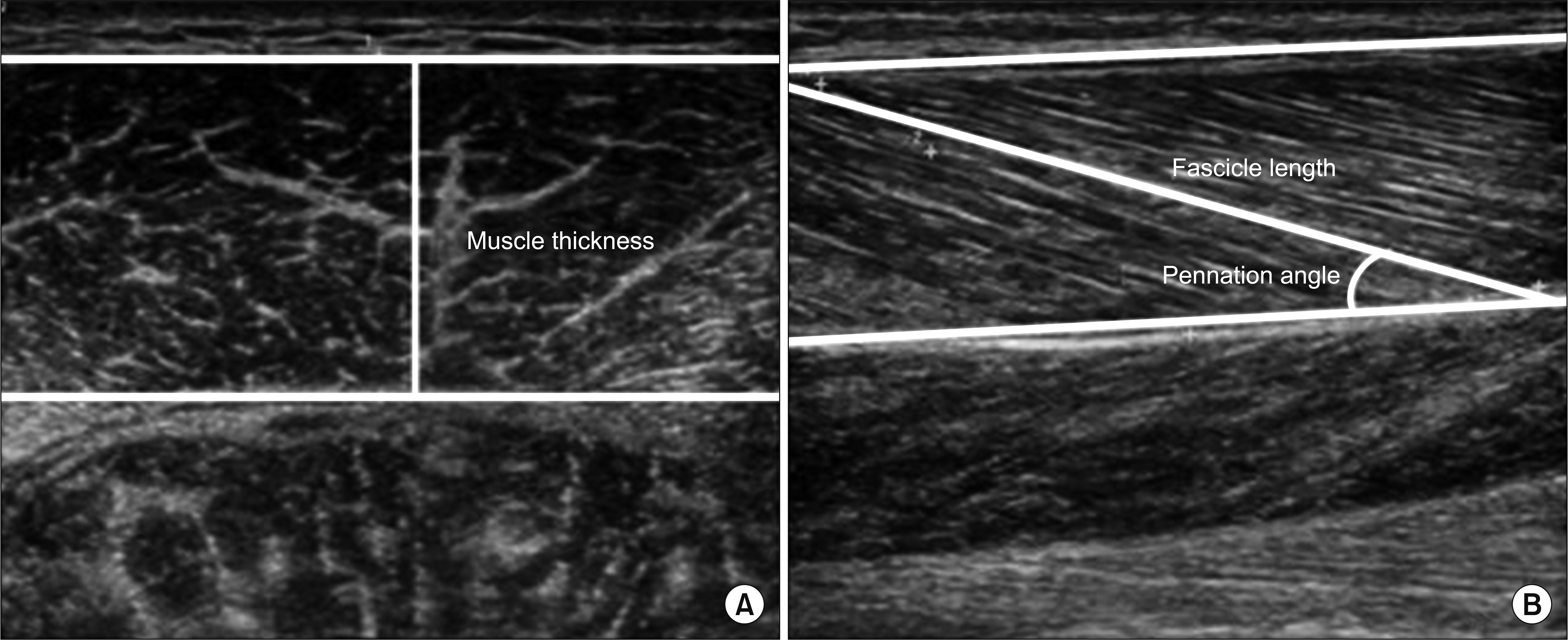
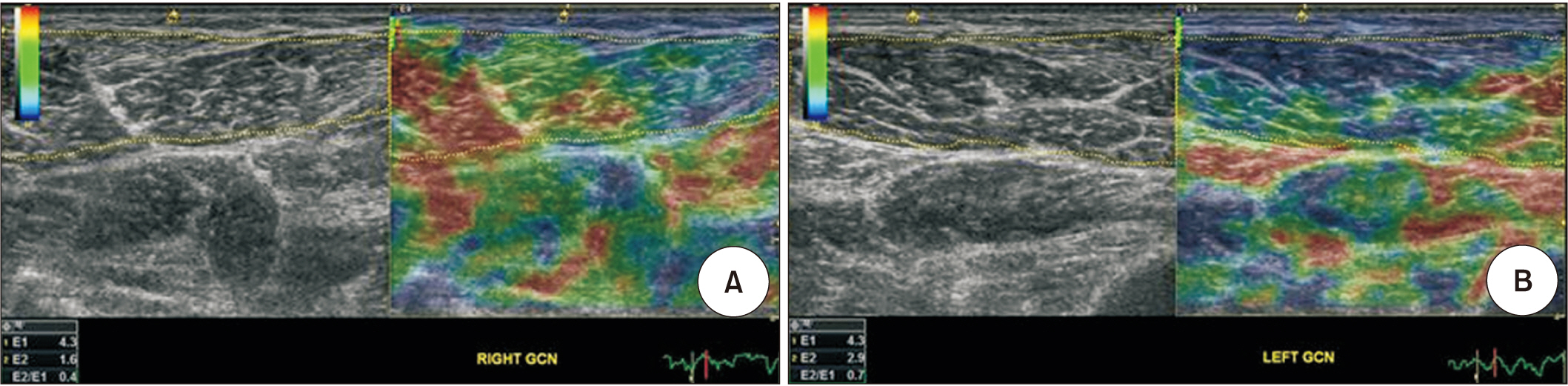
Eight hemiplegic stroke patients with ankle plantarflexor spasticity confirmed by a Modified Ashworth Scale (MAS) were enrolled. Spasticity was evaluated using the MAS and Modified Tardieu Scale (MTS). Each subject underwent surface electromyography (sEMG) using the Brain Motor Control Assessment (BMCA) protocol and was compared with a healthy control group. Using ultrasonography, muscle architecture and elasticity index were measured from the medial gastrocnemius muscle (GCM) on the affected and unaffected sides.
RESULTS
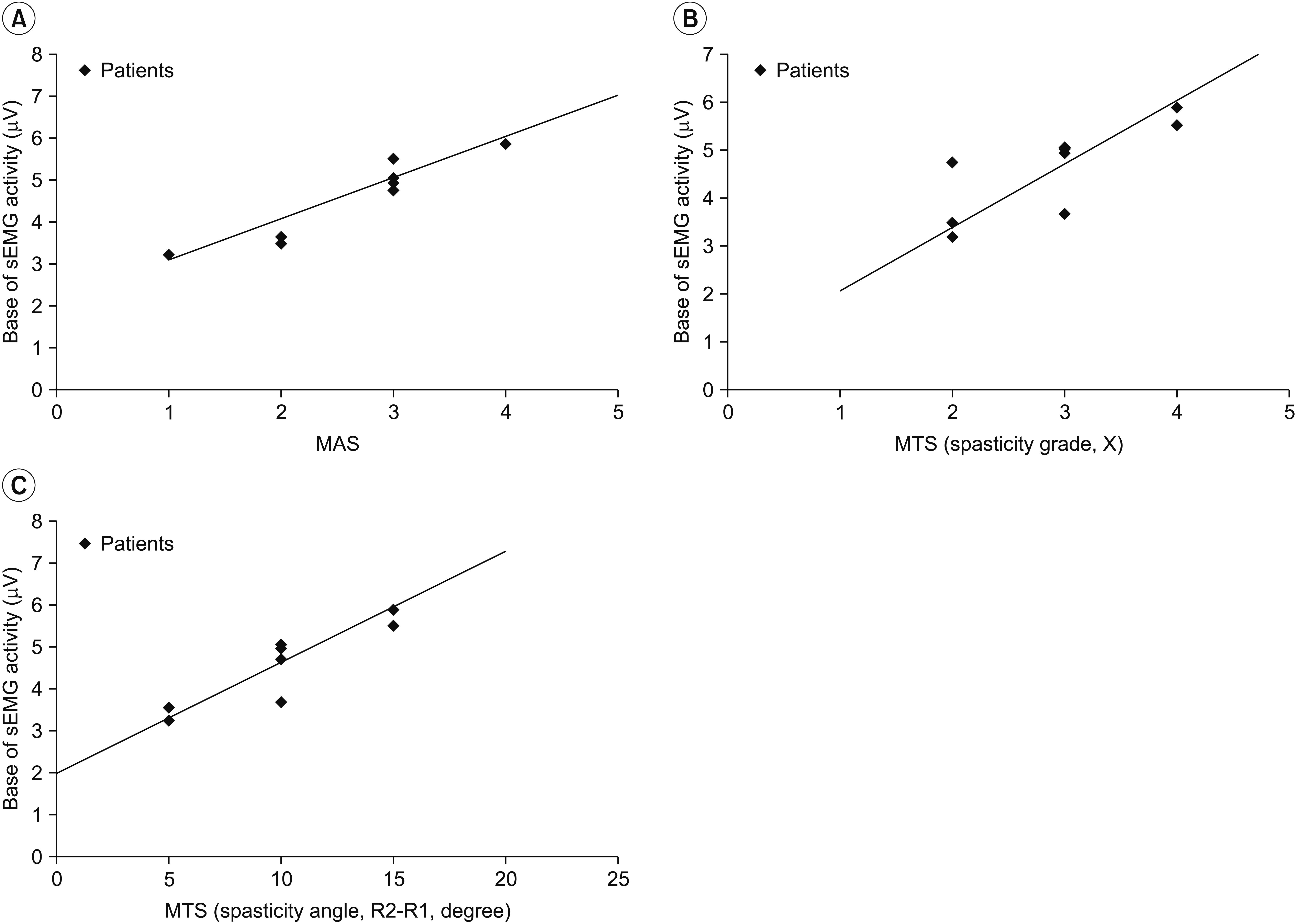
MAS and MTS revealed significant correlation with sEMG activity. The fascicle length and pennation angle were significantly decreased in the medial GCM on the hemiplegic side compared with the unaffected side. The elasticity index of the spastic medial GCM was significantly increased compared with the unaffected side. The MTS X and R2-R1 values were significantly correlated with the elasticity index in the hemiplegic GCM. The relationship between clinical evaluation tools and both BMCA and sonoelastography was linear, but not statistically significant in the multiple regression analysis.
CONCLUSION
The BMCA protocol and ultrasonographic evaluation provide objective assessment of post-stroke spasticity. Further studies are necessary to conduct accurate assessment and treatment of spasticity.
Keyword
MeSH Terms
Figure
Reference
-
1. Lance JW. The control of muscle tone, reflexes, and movement: Robert Wartenberg Lecture. Neurology. 1980; 30:1303–13.2. van Wijck FM, Pandyan AD, Johnson GR, Barnes MP. Assessing motor deficits in neurological rehabilitation: patterns of instrument usage. Neurorehabil Neural Repair. 2001; 15:23–30.
Article3. Li F, Wu Y, Li X. Test-retest reliability and inter-rater reliability of the Modified Tardieu Scale and the Modified Ashworth Scale in hemiplegic patients with stroke. Eur J Phys Rehabil Med. 2014; 50:9–15.4. Alibiglou L, Rymer WZ, Harvey RL, Mirbagheri MM. The relation between Ashworth scores and neuromechanical measurements of spasticity following stroke. J Neuroeng Rehabil. 2008; 5:18.
Article5. Biering-Sorensen F, Nielsen JB, Klinge K. Spasticityassessment: a review. Spinal Cord. 2006; 44:708–22.
Article6. Kreifeldt JG. Signal versus noise characteristics of filtered EMG used as a control source. IEEE Trans Biomed Eng. 1971; 18:16–22.
Article7. Sherwood AM, McKay WB, Dimitrijevic MR. Motor control after spinal cord injury: assessment using surface EMG. Muscle Nerve. 1996; 19:966–79.
Article8. Dimitrijevic MR, Hsu CY, McKay WB. Neurophysiological assessment of spinal cord and head injury. J Neurotrauma. 1992; 9 Suppl 1:S293–300.9. Park GY, Kwon DR. Application of real-time sonoelastography in musculoskeletal diseases related to physical medicine and rehabilitation. Am J Phys Med Rehabil. 2011; 90:875–86.
Article10. Drakonaki EE, Allen GM, Wilson DJ. Ultrasound elastography for musculoskeletal applications. Br J Radiol. 2012; 85:1435–45.
Article11. Lee DC, Lim HK, McKay WB, Priebe MM, Holmes SA, Sherwood AM. Toward an objective interpretation of surface EMG patterns: a voluntary response index (VRI). J Electromyogr Kinesiol. 2004; 14:379–88.
Article12. Sherwood AM, Priebe MM, Graves DE. Consistency of multi-channel surface EMG recordings: application in spinal cord injured subjects. J Electromyogr Kinesiol. 1997; 7:97–111.
Article13. Sherwood AM, Graves DE, Priebe MM. Altered motor control and spasticity after spinal cord injury: subjective and objective assessment. J Rehabil Res Dev. 2000; 37:41–52.14. Zoghi M, Galea M, Morgan D. A Brain Motor Control Assessment (BMCA) protocol for upper limb function. PLoS One. 2013; 8:e79483.
Article15. Kwon DR, Park GY, Lee SU, Chung I. Spastic cerebral palsy in children: dynamic sonoelastographic findings of medial gastrocnemius. Radiology. 2012; 263:794–801.
Article16. Maganaris CN, Baltzopoulos V, Sargeant AJ. In vivo measurements of the triceps surae complex architecture in man: implications for muscle function. J Physiol. 1998; 512(Pt 2):603–14.17. Kesikburun S, Yasar E, Adıguzel E, Guzelkucuk U, Alaca R, Tan AK. Assessment of spasticity with sonoelastography following stroke: a feasibility study. PM R. 2015; 7:1254–60.
Article18. Boyd RN, Graham HK. Objective measurement of clinical findings in the use of botulinum toxin type A for the management of children with cerebral palsy. Eur J Neurol. 1999; 6 Suppl 4:s23–35.
Article19. Mayer NH, Esquenazi A. Muscle overactivity and movement dysfunction in the upper motoneuron syndrome. Phys Med Rehabil Clin N Am. 2003; 14:855–83.
Article20. Wissel J, Schelosky LD, Scott J, Christe W, Faiss JH, Mueller J. Early development of spasticity following stroke: a prospective, observational trial. J Neurol. 2010; 257:1067–72.
Article21. Vattanasilp W, Ada L, Crosbie J. Contribution of thixotropy, spasticity, and contracture to ankle stiffness after stroke. J Neurol Neurosurg Psychiatry. 2000; 69:34–9.
Article22. Thibaut A, Chatelle C, Ziegler E, Bruno MA, Laureys S, Gosseries O. Spasticity after stroke: physiology, assessment and treatment. Brain Inj. 2013; 27:1093–105.
Article23. Shortland AP, Harris CA, Gough M, Robinson RO. Architecture of the medial gastrocnemius in children with spastic diplegia. Dev Med Child Neurol. 2002; 44:158–63.
Article24. Sherwood AM, Dimitrijevic MR, McKay WB. Evidence of subclinical brain influence in clinically complete spinal cord injury: discomplete SCI. J Neurol Sci. 1992; 110:90–8.
Article25. McKay WB, Lim HK, Priebe MM, Stokic DS, Sherwood AM. Clinical neurophysiological assessment of residual motor control in post-spinal cord injury paralysis. Neurorehabil Neural Repair. 2004; 18:144–53.
Article26. Legerlotz K, Smith HK, Hing WA. Variation and reliability of ultrasonographic quantification of the architecture of the medial gastrocnemius muscle in young children. Clin Physiol Funct Imaging. 2010; 30:198–205.
Article27. Gao F, Grant TH, Roth EJ, Zhang LQ. Changes in passive mechanical properties of the gastrocnemius muscle at the muscle fascicle and joint levels in stroke survivors. Arch Phys Med Rehabil. 2009; 90:819–26.
Article28. Friden J, Lieber RL. Spastic muscle cells are shorter and stiffer than normal cells. Muscle Nerve. 2003; 27:157–64.
Article29. Yasar E, Adiguzel E, Kesikburun S, Yenihayat I, Yilmaz B, Alaca R, et al. Assessment of forearm muscle spasticity with sonoelastography in patients with stroke. Br J Radiol. 2016; 89:20160603.30. Allison SC, Abraham LD, Petersen CL. Reliability of the Modified Ashworth Scale in the assessment of plantarflexor muscle spasticity in patients with traumatic brain injury. Int J Rehabil Res. 1996; 19:67–78.
Article31. Vattanasilp W, Ada L. The relationship between clinical and laboratory measures of spasticity. Aust J Physiother. 1999; 45:135–9.
Article32. Feldman RG, Young RR, Koella WP. Spasticity, disordered motor control. Chicago: Year Book Medical Publishers;1980. p. 485–500.33. Dietz V, Sinkjaer T. Spastic movement disorder: impaired reflex function and altered muscle mechanics. Lancet Neurol. 2007; 6:725–33.
Article34. Kawakami Y, Ichinose Y, Fukunaga T. Architectural and functional features of human triceps surae muscles during contraction. J Appl Physiol (1985). 1998; 85:398–404.35. Dias CP, Freire B, Goulart NB, Onzi ES, Becker J, Gomes I, et al. Muscle architecture and torque production in stroke survivors: an observational study. Top Stroke Rehabil. 2017; 24:206–13.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Quantitative Assessment of the Effect of Tizanidine on Spasticity in Stroke Patients
- Extracorporeal Shock Wave Therapy and Quantitative Ultrasonographic Evaluation of the Rheologic Effect in the Patients with Post-stroke Upper Limb Spasticity: A Case Report
- Effectiveness of a home-based exercise program among patients with lower limb spasticity post-stroke: A randomized controlled trial
- Ultrasound-Guided Botulinum Toxin Injection with Factor VIII Administration for Post Stroke Spasticity in a Hemophilia A Patient
- The Effect of a Hand-Stretching Device During the Management of Spasticity in Chronic Hemiparetic Stroke Patients