Yonsei Med J.
2018 Aug;59(6):787-792. 10.3349/ymj.2018.59.6.787.
Effect of rs3910105 in the Synuclein Gene on Dopamine Transporter Availability in Healthy Subjects
- Affiliations
-
- 1Department of Nuclear Medicine, Busan Seongso Hospital, Busan, Korea.
- 2Department of Nuclear Medicine and Biomedical Research Institute, Pusan National University Hospital, Busan, Korea. ilikechopin@me.com
- 3Department of Nuclear Medicine, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea.
- 4Department of Nuclear Medicine, Chung-Ang University College of Medicine, Seoul, Korea. ethmoid@daum.net
- 5Department of Neurology and Biomedical Research Institute, Pusan National University Hospital, Busan, Korea.
- 6Department of Neurosurgery and Biomedical Research Institute, Pusan National University Hospital, Busan, Korea.
- 7Department of Nuclear Medicine and Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Yangsan, Korea.
- KMID: 2415538
- DOI: http://doi.org/10.3349/ymj.2018.59.6.787
Abstract
- PURPOSE
The present study investigated associations between dopamine transporter (DAT) availability and α-synuclein levels in cerebrospinal fluid, as well as synuclein gene (SNCA) transcripts, and the effect of single nucleotide polymorphism of SNCA on DAT availability in healthy subjects.
MATERIALS AND METHODS
The study population comprised healthy controls who underwent 123I-FP-CIT single-photon emission computed tomography screening. Five SNCA probes were used to target the boundaries of exon 3 and exon 4 (SNCA-E3E4), transcripts with a long 3"²UTR region (SNCA-3UTR-1, SNCA-3UTR-2), transcripts that skip exon 5 (SNCA-E4E6), and the rare short transcript isoforms that comprise exons 1-4 (SNCA-007).
RESULTS
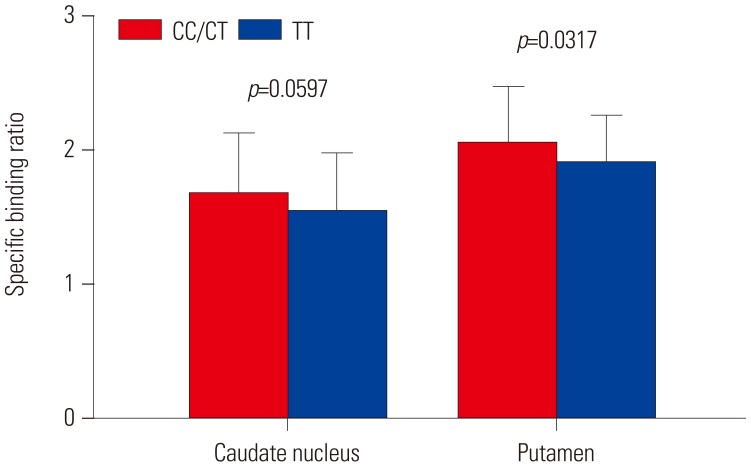
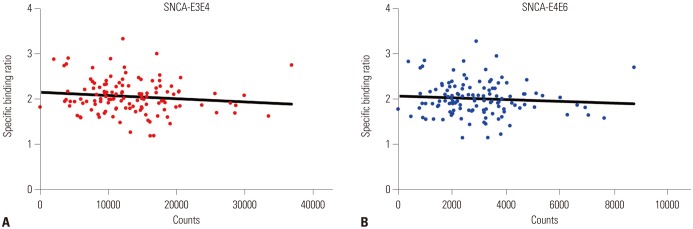
In total, 123 healthy subjects (male 75, female 48) were included in this study. DAT availability in the caudate nucleus (p=0.0661) and putamen (p=0.0739) tended to differ according to rs3910105 genotype. In post-hoc analysis, DAT availability in the putamen was lower in subjects of TT genotype than those of CC/CT (p=0.0317). DAT availability in the caudate nucleus also showed a trend similar to that in the putamen (p=0.0597). Subjects of CT genotype with rs3910105 showed negative correlations with DAT availability in the putamen with SNCA-E3E4 (p=0.037, rho=−0.277), and SNCA-E4E6 (p=0.042, rho=−0.270), but not those of CC/TT genotypes.
CONCLUSION
This is the first study to investigate the association of rs3910105 in SNCA with DAT availability. rs3910105 had an effect on DAT availability, and the correlation between DAT availability and SNCA transcripts were significant in CT genotypes of rs3910105.
Keyword
MeSH Terms
-
Biomarkers
Caudate Nucleus
Cerebrospinal Fluid
Dopamine Plasma Membrane Transport Proteins*
Dopamine*
Exons
Female
Genotype
Healthy Volunteers*
Humans
Mass Screening
Polymorphism, Single Nucleotide
Protein Isoforms
Putamen
Synucleins*
Tomography, Emission-Computed
Biomarkers
Dopamine
Dopamine Plasma Membrane Transport Proteins
Protein Isoforms
Synucleins
Figure
Reference
-
1. Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies. Proc Natl Acad Sci U S A. 1998; 95:6469–6473. PMID: 9600990.2. Politis M. Neuroimaging in Parkinson disease: from research setting to clinical practice. Nat Rev Neurol. 2014; 10:708–722. PMID: 25385334.
Article3. Park E. A new era of clinical dopamine transporter imaging using 123I-FP-CIT. J Nucl Med Technol. 2012; 40:222–228. PMID: 23160562.
Article4. Booth TC, Nathan M, Waldman AD, Quigley AM, Schapira AH, Buscombe J. The role of functional dopamine-transporter SPECT imaging in parkinsonian syndromes, part 1. AJNR Am J Neuroradiol. 2015; 36:229–235. PMID: 24904053.
Article5. Marshall V, Grosset D. Role of dopamine transporter imaging in routine clinical practice. Mov Disord. 2003; 18:1415–1423. PMID: 14673877.
Article6. Zipursky RB, Meyer JH, Verhoeff NP. PET and SPECT imaging in psychiatric disorders. Can J Psychiatry. 2007; 52:146–157. PMID: 17479522.
Article7. Thomas AJ, Attems J, Colloby SJ, O'Brien JT, McKeith I, Walker R, et al. Autopsy validation of 123I-FP-CIT dopaminergic neuroimaging for the diagnosis of DLB. Neurology. 2017; 88:276–283. PMID: 27940650.
Article8. Koch W, Unterrainer M, Xiong G, Bartenstein P, Diemling M, Varrone A, et al. Extrastriatal binding of [123I]FP-CIT in the thalamus and pons: gender and age dependencies assessed in a European multicentre database of healthy controls. Eur J Nucl Med Mol Imaging. 2014; 41:1938–1946. PMID: 24806112.9. Stefanis L. α-Synuclein in Parkinson's disease. Cold Spring Harb Perspect Med. 2012; 2:a009399. PMID: 22355802.
Article10. Beyer K. Alpha-synuclein structure, posttranslational modification and alternative splicing as aggregation enhancers. Acta Neuropathol. 2006; 112:237–251. PMID: 16845533.11. Bisaglia M, Mammi S, Bubacco L. Structural insights on physiological functions and pathological effects of alpha-synuclein. FASEB J. 2009; 23:329–340. PMID: 18948383.12. Sidhu A, Wersinger C, Vernier P. alpha-Synuclein regulation of the dopaminergic transporter: a possible role in the pathogenesis of Parkinson's disease. FEBS Lett. 2004; 565:1–5. PMID: 15135042.13. Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ. A role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci. 2002; 22:3090–3099. PMID: 11943812.14. Spillantini MG, Goedert M. The alpha-synucleinopathies: Parkinson's disease, dementia with Lewy bodies, and multiple system atrophy. Ann N Y Acad Sci. 2000; 920:16–27. PMID: 11193145.15. Braak H, Del Tredici K, Bratzke H, Hamm-Clement J, Sandmann-Keil D, Rüb U. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson's disease (preclinical and clinical stages). J Neurol. 2002; 249(Suppl 3):III/1–III/5.
Article16. Mollenhauer B, Locascio JJ, Schulz-Schaeffer W, Sixel-Döring F, Trenkwalder C, Schlossmacher MG. α-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: a cohort study. Lancet Neurol. 2011; 10:230–240. PMID: 21317042.
Article17. Beyer K, Domingo-Sábat M, Lao JI, Carrato C, Ferrer I, Ariza A. Identification and characterization of a new alpha-synuclein isoform and its role in Lewy body diseases. Neurogenetics. 2008; 9:15–23. PMID: 17955272.
Article18. Gao R, Zhang G, Chen X, Yang A, Smith G, Wong DF, et al. CSF biomarkers and its associations with 18F-AV133 cerebral VMAT2 binding in Parkinson's disease-a preliminary report. PLoS One. 2016; 11:e0164762. PMID: 27764160.
Article19. Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997; 388:839–840. PMID: 9278044.20. Edwards TL, Scott WK, Almonte C, Burt A, Powell EH, Beecham GW, et al. Genome-wide association study confirms SNPs in SNCA and the MAPT region as common risk factors for Parkinson disease. Ann Hum Genet. 2010; 74:97–109. PMID: 20070850.21. Parkinson Progression Marker Initiative. The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol. 2011; 95:629–635. PMID: 21930184.22. García-Gómez FJ, García-Solís D, Luis-Simón FJ, Marín-Oyaga VA, Carrillo F, Mir P, et al. [Elaboration of the SPM template for the standardization of SPECT images with 123I-Ioflupane]. Rev Esp Med Nucl Imagen Mol. 2013; 32:350–356. PMID: 23570700.
Article23. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002; 15:273–289. PMID: 11771995.
Article24. Mollenhauer B, Caspell-Garcia CJ, Coffey CS, Taylor P, Shaw LM, Trojanowski JQ, et al. Longitudinal CSF biomarkers in patients with early Parkinson disease and healthy controls. Neurology. 2017; 89:1959–1969. PMID: 29030452.
Article25. Heckman MG, Kasanuki K, Diehl NN, Koga S, Soto A, Murray ME, et al. Parkinson's disease susceptibility variants and severity of Lewy body pathology. Parkinsonism Relat Disord. 2017; 44:79–84. PMID: 28917824.
Article26. Caspell-Garcia C, Simuni T, Tosun-Turgut D, Wu IW, Zhang Y, Nalls M, et al. Multiple modality biomarker prediction of cognitive impairment in prospectively followed de novo Parkinson disease. PLoS One. 2017; 12:e0175674. PMID: 28520803.
Article27. Chen PS, Yeh TL, Lee IH, Lin CB, Tsai HC, Chen KC, et al. Effects of C825T polymorphism of the GNB3 gene on availability of dopamine transporter in healthy volunteers--a SPECT study. Neuroimage. 2011; 56:1526–1530. PMID: 21371559.28. van de Giessen E, de Win MM, Tanck MW, van den Brink W, Baas F, Booij J. Striatal dopamine transporter availability associated with polymorphisms in the dopamine transporter gene SLC6A3. J Nucl Med. 2009; 50:45–52. PMID: 19091889.
Article29. van Dyck CH, Malison RT, Jacobsen LK, Seibyl JP, Staley JK, Laruelle M, et al. Increased dopamine transporter availability associated with the 9-repeat allele of the SLC6A3 gene. J Nucl Med. 2005; 46:745–751. PMID: 15872345.30. Fazio L, Blasi G, Taurisano P, Papazacharias A, Romano R, Gelao B, et al. D2 receptor genotype and striatal dopamine signaling predict motor cortical activity and behavior in humans. Neuroimage. 2011; 54:2915–2921. PMID: 21087673.
Article31. Locascio JJ, Eberly S, Liao Z, Liu G, Hoesing AN, Duong K, et al. Association between α-synuclein blood transcripts and early, neuroimaging-supported Parkinson's disease. Brain. 2015; 138(Pt 9):2659–2671. PMID: 26220939.
Article32. Emamzadeh FN. Alpha-synuclein structure, functions, and interactions. J Res Med Sci. 2016; 21:29. PMID: 27904575.
Article33. Wong YC, Krainc D. α-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat Med. 2017; 23:1–13.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Single-Nucleotide Polymorphisms on Decline of Dopamine Transporter Availability in Parkinson's Disease
- Classic Studies on the Interaction of Cocaine and the Dopamine Transporter
- Loss of Striatal Dopamine Transporter Binding in Patients and a Family Member with Familial Parkinsonism with Parkin Gene Mutation
- Dopamine Transporter Gene Polymorphism In ADHD
- Lack of Association between Polymorphisms of the Dopamine Receptor D4 and Dopamine Transporter Genes and Personality Traits in a Korean Population