J Clin Neurol.
2018 Jul;14(3):393-400. 10.3988/jcn.2018.14.3.393.
Postoperative Transient Neurologic Dysfunction: A Proposal for Pathophysiology
- Affiliations
-
- 1Division of Pediatric Neurosurgery, Seoul National University Children's Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 2Department of Neurosurgery, Kangwon National University School of Medicine, Chuncheon, Korea.
- 3Department of Neurosurgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea. nsdrcho@gmail.com
- 4Department of Neurology, Kangwon National University School of Medicine, Chuncheon, Korea. leeseoyoung@kangwon.ac.kr
- KMID: 2415058
- DOI: http://doi.org/10.3988/jcn.2018.14.3.393
Abstract
- BACKGROUND AND PURPOSE
Sudden neurological deterioration which cannot be explained by structural change, ischemia or seizure is often observed among neurosurgical patients. We aimed to provide new insight into the pathophysiology of postoperative transient neurologic dysfunction.
METHODS
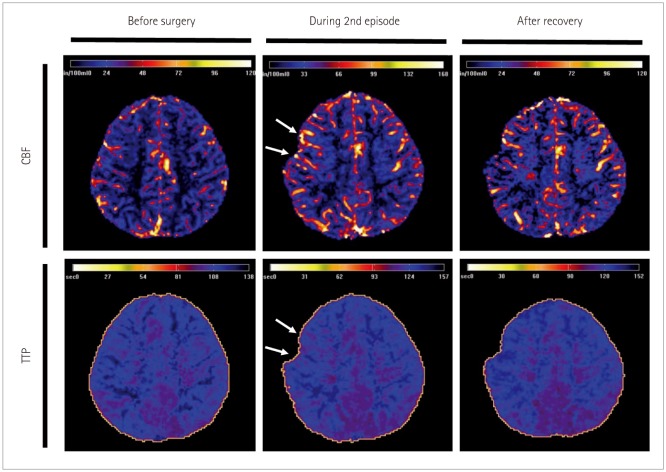
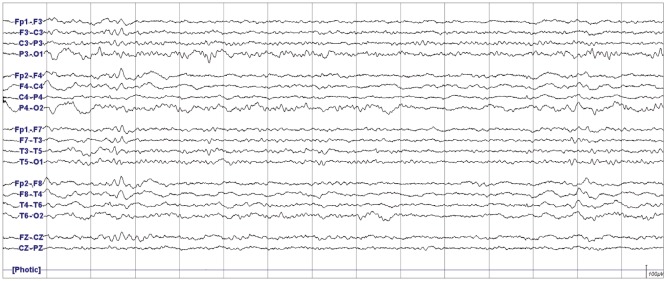
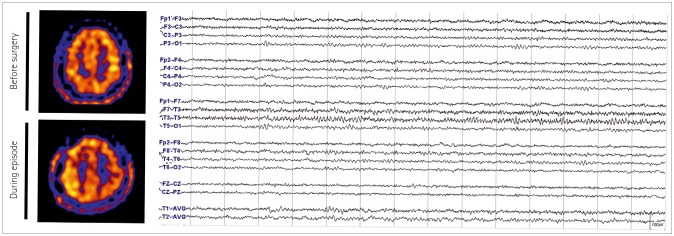
We describe prolonged but fully reversible focal neurologic dysfunction of unknown origin based on the initial evaluation in 8 patients who had received encephalo-duro-arterio-synangiosis for moyamoya disease. We performed brain imaging, including diffusion weighted imaging and perfusion magnetic resonance imaging or single photon emission computed tomography, and electroencephalography (EEG) during the episodes and after resolution of the symptoms.
RESULTS
The symptoms consisted of dysarthria, hemiparesis, or hemiparesthesia of limbs contralateral to the operated side. These symptoms developed between 12 hours and 8 days after surgery and lasted between 12 hours and 17 days. Structural imaging did not show any significant interval change compared with the immediate postoperative images. Perfusion imaging showed increased cerebral blood flow in the symptomatic hemisphere. EEG revealed low amplitude arrhythmic slowing in the corresponding hemisphere. Follow-up imaging and EEG after recovery did not show any abnormalities.
CONCLUSIONS
Transient neurologic dysfunction can occur during the postoperative period of brain surgery. Although this may last more than usual transient ischemic attack or seizure, it eventually resolves regardless of treatment. Based on our observation, we propose that this is the manifestation of the transient cortical depression triggered by mechanical stimulation, analogous to migraine aura associated with cortical spreading depression.
Keyword
MeSH Terms
-
Brain
Cerebrovascular Circulation
Cortical Spreading Depression
Depression
Diffusion
Dysarthria
Electroencephalography
Epilepsy
Extremities
Follow-Up Studies
Humans
Ischemia
Ischemic Attack, Transient
Magnetic Resonance Angiography
Migraine Disorders
Moyamoya Disease
Neuroimaging
Neurologic Manifestations*
Paresis
Perfusion Imaging
Postoperative Period
Seizures
Tomography, Emission-Computed, Single-Photon
Figure
Reference
-
1. Leão AA. Spreading depression of activity in the cerebral cortex. J Neurophysiol. 1944; 7:359–390.2. Somjen GG. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol Rev. 2001; 81:1065–1096. PMID: 11427692.3. Strong AJ, Fabricius M, Boutelle MG, Hibbins SJ, Hopwood SE, Jones R, et al. Spreading and synchronous depressions of cortical activity in acutely injured human brain. Stroke. 2002; 33:2738–2743. PMID: 12468763.
Article4. Mayevsky A, Doron A, Manor T, Meilin S, Zarchin N, Ouaknine GE. Cortical spreading depression recorded from the human brain using a multiparametric monitoring system. Brain Res. 1996; 740:268–274. PMID: 8973824.
Article5. Hartings JA, Bullock MR, Okonkwo DO, Murray LS, Murray GD, Fabricius M, et al. Spreading depolarisations and outcome after traumatic brain injury: a prospective observational study. Lancet Neurol. 2011; 10:1058–1064. PMID: 22056157.
Article6. Dohmen C, Sakowitz OW, Fabricius M, Bosche B, Reithmeier T, Ernestus RI, et al. Spreading depolarizations occur in human ischemic stroke with high incidence. Ann Neurol. 2008; 63:720–728. PMID: 18496842.
Article7. Fabricius M, Fuhr S, Bhatia R, Boutelle M, Hashemi P, Strong AJ, et al. Cortical spreading depression and peri-infarct depolarization in acutely injured human cerebral cortex. Brain. 2006; 129:778–790. PMID: 16364954.
Article8. Cho WS, Kim JE, Kim CH, Ban SP, Kang HS, Son YJ, et al. Long-term outcomes after combined revascularization surgery in adult moyamoya disease. Stroke. 2014; 45:3025–3031. PMID: 25184359.
Article9. Pietrobon D, Striessnig J. Neurobiology of migraine. Nat Rev Neurosci. 2003; 4:386–398. PMID: 12728266.
Article10. Russell MB, Ducros A. Sporadic and familial hemiplegic migraine: pathophysiological mechanisms, clinical characteristics, diagnosis, and management. Lancet Neurol. 2011; 10:457–470. PMID: 21458376.
Article11. Choi KH, Kim JS, Lee SY, Ryu SW, Kim SS, Lee SH, et al. Familial hemiplegic migraine with prolonged coma and cerebellar atrophy: CACNA1A T666M mutation in a Korean family. J Korean Med Sci. 2012; 27:1124–1127. PMID: 22969264.
Article12. Fujimura M, Kaneta T, Shimizu H, Tominaga T. Symptomatic hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in a child with moyamoya disease. Childs Nerv Syst. 2007; 23:1195–1198. PMID: 17486352.
Article13. Kim JE, Oh CW, Kwon OK, Park SQ, Kim SE, Kim YK. Transient hyperperfusion after superficial temporal artery/middle cerebral artery bypass surgery as a possible cause of postoperative transient neurological deterioration. Cerebrovasc Dis. 2008; 25:580–586. PMID: 18483458.
Article14. Cho WS, Lee HY, Kang HS, Kim JE, Bang JS, Oh CW. Symptomatic cerebral hyperperfusion on SPECT after indirect revascularization surgery for moyamoya disease. Clin Nucl Med. 2013; 38:44–46. PMID: 23242046.
Article15. Rajapakse T, Buchhalter J. The borderland of migraine and epilepsy in children. Headache. 2016; 56:1071–1080. PMID: 27103497.
Article16. Haas DC, Lourie H. Trauma-triggered migraine: an explanation for common neurological attacks after mild head injury. review of the literature. J Neurosurg. 1988; 68:181–188. PMID: 3276835.17. Sakas DE, Whittaker KW, Whitwell HL, Singounas EG. Syndromes of posttraumatic neurological deterioration in children with no focal lesions revealed by cerebral imaging: evidence for a trigeminovascular pathophysiology. Neurosurgery. 1997; 41:661–667. PMID: 9310985.
Article18. John Graham senior clinicians award lecture. posttraumatic migraine. Headache. 1998; 38:772–778. PMID: 11279902.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Successful Treatment of Thrombotic Thrombocytopenic Purpura with Recurrent Transient Ischemic Attacks in Old Age
- Acquired Adult Flatfoot: Pathophysiology, Diagnosis, and Nonoperative Treatment
- Transient Left Ventricular Systolic Dysfunction Associated with Carbon Monoxide Toxicity
- Pathophysiology and Evaluation of Upper Esophageal Dysfunction
- Transient Global Amnesia Developed in Recovery Room following General Anesthesia: A case report