J Vet Sci.
2016 Jun;17(2):171-178. 10.4142/jvs.2016.17.2.171.
Downregulation of cellular prion protein inhibited the proliferation and invasion and induced apoptosis of Marek's disease virus-transformed avian T cells
- Affiliations
-
- 1Department of Preventive Veterinary, College of Veterinary Medicine, Gansu Agricultural University, Lanzhou 730070, China. wurun@gsau.edu.cn
- KMID: 2413166
- DOI: http://doi.org/10.4142/jvs.2016.17.2.171
Abstract
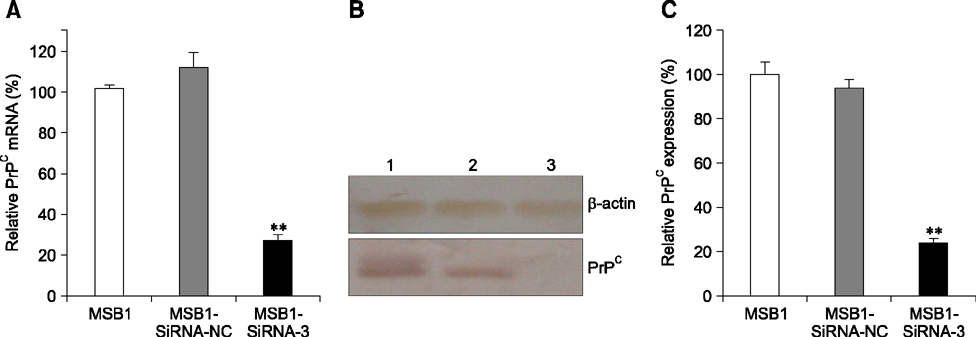
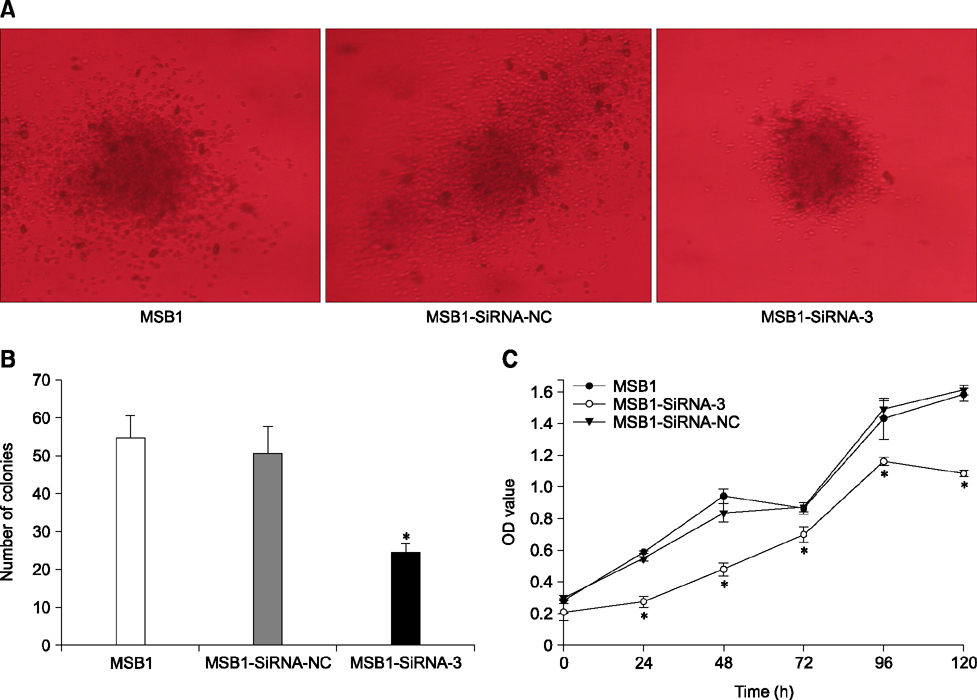
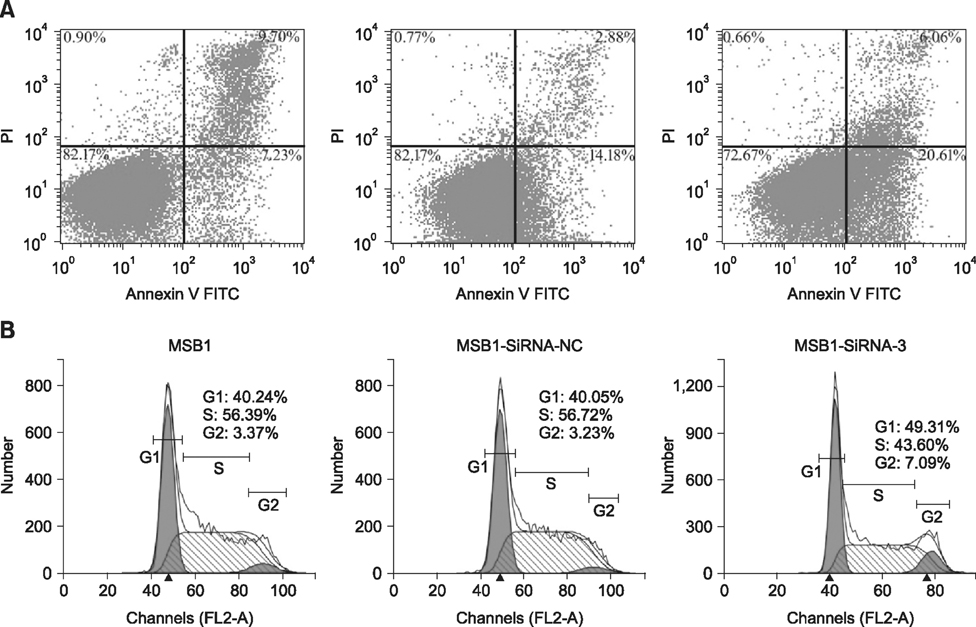
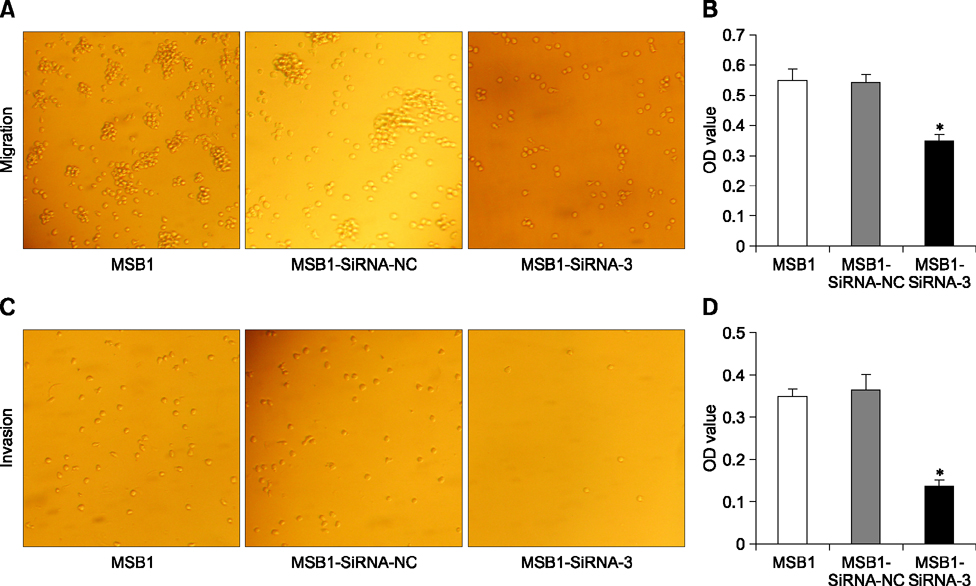
- Cellular prion protein (PrP(C)) is ubiquitously expressed in the cytomembrane of a considerable number of eukaryotic cells. Although several studies have investigated the functions of PrP(C) in cell proliferation, cell apoptosis, and tumorigenesis of mammals, the correlated functions of chicken PrP(C) (chPrP(C)) remain unknown. In this study, stable chPrP(C)-downregulated Marek's disease (MD) virus-transformed avian T cells (MSB1-SiRNA-3) were established by introducing short interfering RNA (SiRNA) targeting chicken prion protein genes. We found that downregulation of chPrP(C) inhibits proliferation, invasion, and migration, and induces G1 cell cycle phase arrest and apoptosis of MSB1-SiRNA-3 cells compared with Marek's disease virus-transformed avian T cells (MSB1) and negative control cells. To the best of our knowledge, the present study provides the first evidence supporting the positive correlation between the expression level of chPrP(C) and the proliferation, migration, and invasion ability of MSB1 cells, but appears to protect MSB1 cells from apoptosis, which suggests it functions in the formation and development of MD tumors. This evidence may contribute to future research into the specific molecular mechanisms of chPrP(C) in the formation and development of MD tumors.
Keyword
MeSH Terms
-
Animals
*Apoptosis/genetics
Cell Cycle
Cell Line
Cell Movement
Cell Proliferation/genetics
Cell Transformation, Viral
*Chickens
*Down-Regulation
Herpesvirus 2, Gallid/*physiology
Marek Disease/virology
Poultry Diseases/*virology
Prion Proteins/*genetics/metabolism
RNA, Small Interfering/genetics/metabolism
T-Lymphocytes/*physiology/virology
Prion Proteins
RNA, Small Interfering
Figure
Reference
-
1. Ai WN, Wu R, Diao XL. Development and primary application of double-standard curves method of a real-time fluorescent quantitative PCR assay for detection of PRNP. Chin Vet Sci. 2013; 43:881–886.2. Akiyama Y, Kato S. Two cell lines from lymphomas of Marek's disease. Biken J. 1974; 17:105–116.3. Bounhar Y, Zhang Y, Goodyer CG, LeBlanc A. Prion protein protects human neurons against Bax-mediated apoptosis. J Biol Chem. 2001; 276:39145–39149.
Article4. Brown DR, Clive C, Haswell SJ. Antioxidant activity related to copper binding of native prion protein. J Neurochem. 2001; 76:69–76.
Article5. Calnek BW. Marek's disease–a model for herpesvirus oncology. Crit Rev Microbiol. 1986; 12:293–320.
Article6. Diao XL, Wu R, Liu L, Zhao CL, Wang X, Guan HW, Luo P. Expression patterns of PrP gene during chicken embryo development. Asian J Anim Vet Adv. 2012; 7:199–204.
Article7. Erdogan S, Duzguner V, Kucukgul A, Aslantas O. Silencing of PrPC (prion protein) expression does not affect Brucella melitensis infection in human derived microglia cells. Res Vet Sci. 2013; 95:368–373.
Article8. Fan Q, Wang X, Zhang H, Li C, Fan J, Xu J. Silencing cathepsin S gene expression inhibits growth, invasion and angiogenesis of human hepatocellular carcinoma in vitro. Biochem Biophys Res Commun. 2012; 425:703–710.
Article9. Green DR, Evan GI. A matter of life and death. Cancer Cell. 2002; 1:19–30.
Article10. Ji HF, Zhang HY. A comparative molecular dynamics study on thermostability of human and chicken prion proteins. Biochem Biophys Res Commun. 2007; 359:790–794.
Article11. Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002; 108:153–164.12. Kang SG, Roh YM, Lau A, Westaway D, McKenzie D, Aiken J, Kim YS, Yoo HS. Establishment and characterization of Prnp knockdown neuroblastoma cells using dual microRNA-mediated RNA interference. Prion. 2011; 5:93–102.
Article13. Luo P, Wu R, Diao XL, Guan HW, Zhang L. Prokaryotic expression of chicken prion protein and preparation of its antiserum. Chin Vet Sci. 2012; 42:268–274.14. Mehrpour M, Codogno P. Prion protein: from physiology to cancer biology. Cancer Lett. 2010; 290:1–23.
Article15. Pan Y, Zhao L, Liang J, Liu J, Shi Y, Liu Z, Zhang G, Jin H, Gao J, Xie H, Wang J, Liu Z, Fan D. Cellular prion protein promotes invasion and metastasis of gastric cancer. FASEB J. 2006; 20:E1205–E1215. 1886–1888.
Article16. Peralta OA, Huckle WR, Eyestone WH. Expression and knockdown of cellular prion protein (PrPC) in differentiating mouse embryonic stem cells. Differentiation. 2011; 81:68–77.
Article17. Pham N, Dhar A, Khalaj S, Desai K, Taghibiglou C. Down regulation of brain cellular prion protein in an animal model of insulin resistance: possible implication in increased prevalence of stroke in pre-diabetics/diabetics. Biochem Biophys Res Commun. 2014; 448:151–156.
Article18. Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982; 216:136–144.
Article19. Rachidi W, Mangé A, Senator A, Guiraud P, Riondel J, Benboubetra M, Favier A, Lehmann S. Prion infection impairs copper binding of cultured cells. J Biol Chem. 2003; 278:14595–14598.
Article20. Redecke L, Meyer-Klaucke W, Koker M, Clos J, Georgieva D, Genov N, Echner H, Kalbacher H, Perbandt M, Bredehorst R, Voelter W, Betzel C. Comparative analysis of the human and chicken prion protein copper binding regions at pH 6.5. J Biol Chem. 2005; 280:13987–13992.
Article21. Steele AD, Emsley JG, Özdinler PH, Lindquist S, Macklis JD. Prion protein (PrPC) positively regulates neural precursor proliferation during developmental and adult mammalian neurogenesis. Proc Natl Acad Sci U S A. 2006; 103:3416–3421.
Article22. Thackray AM, Bujdoso R. Elevated PrPC expression predisposes to increased HSV-1 pathogenicity. Antivir Chem Chemother. 2006; 17:41–52.
Article23. Wang XT, Xie YB, Xiao Q. siRNA targeting of Cdx2 inhibits growth of human gastric cancer MGC-803 cells. World J Gastroenterol. 2012; 18:1903–1914.
Article24. Watanabe T, Yasutaka Y, Nishioku T, Kusakabe S, Futagami K, Yamauchi A, Kataoka Y. Involvement of the cellular prion protein in the migration of brain microvascular endothelial cells. Neurosci Lett. 2011; 496:121–124.
Article25. Yap YH, Say YH. Resistance against apoptosis by the cellular prion protein is dependent on its glycosylation status in oral HSC-2 and colon LS 174T cancer cells. Cancer Lett. 2011; 306:111–119.
Article26. Zamore PD, Tuschl T, Sharp PA, Bartel DP. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell. 2000; 101:25–33.27. Zhang Y, Qin K, Wang J, Hung T, Zhao RY. Dividing roles of prion protein in staurosporine-mediated apoptosis. Biochem Biophys Res Commun. 2006; 349:759–768.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- MicroRNA-98-5p Inhibits Tumorigenesis of Hepatitis B Virus-Related Hepatocellular Carcinoma by Targeting NF-κB-Inducing Kinase
- Cross-linking of CD80 and CD86 Diminishes Expression of CD54 on EBV-transformed B Cells through Inactivation of RhoA and Ras
- Cellular Prion Protein Enhances Drug Resistance of Colorectal Cancer Cells via Regulation of a Survival Signal Pathway
- Effects of cyclosporin A treatment on the pathogenesis of avian leukosis virus subgroup J infection in broiler chickens with Marek's disease virus exposure
- Characterization and localization of the unique Marek's disease virus type 2 ORF873 gene product