Immune Netw.
2011 Dec;11(6):390-398. 10.4110/in.2011.11.6.390.
Cross-linking of CD80 and CD86 Diminishes Expression of CD54 on EBV-transformed B Cells through Inactivation of RhoA and Ras
- Affiliations
-
- 1Department of Anatomy and Research Center for Tumor Immunology, Inje University College of Medicine, Busan 614-735, Korea. dyhur@inje.ac.kr
- 2Department of Anatomy, College of Medicine, Seoul National University, Seoul 151-742, Korea.
- KMID: 2150725
- DOI: http://doi.org/10.4110/in.2011.11.6.390
Abstract
- BACKGROUND
Epstein Barr virus (EBV) infected B cells are transformed into lymphoblastoid cell lines. Some researchers suggested some a few similarities between this process and carcinogenesis. We observed the expression of CD80 and CD86, co-stimulatory molecules on EBV-transformed B cells and changes of CD54 expression after stimulation of CD80 and CD86.
METHODS
CD80 and CD86 were stimulated using anti-CD80 and anti-CD86 monoclonal antibodies. To assess apoptosis and surface protein expression, flow cytometric analysis was performed. Intracellular signal molecules were evaluated by RT-PCR and immunoblot. Morphology and localization of proteins were examined using inverted or confocal microscope.
RESULTS
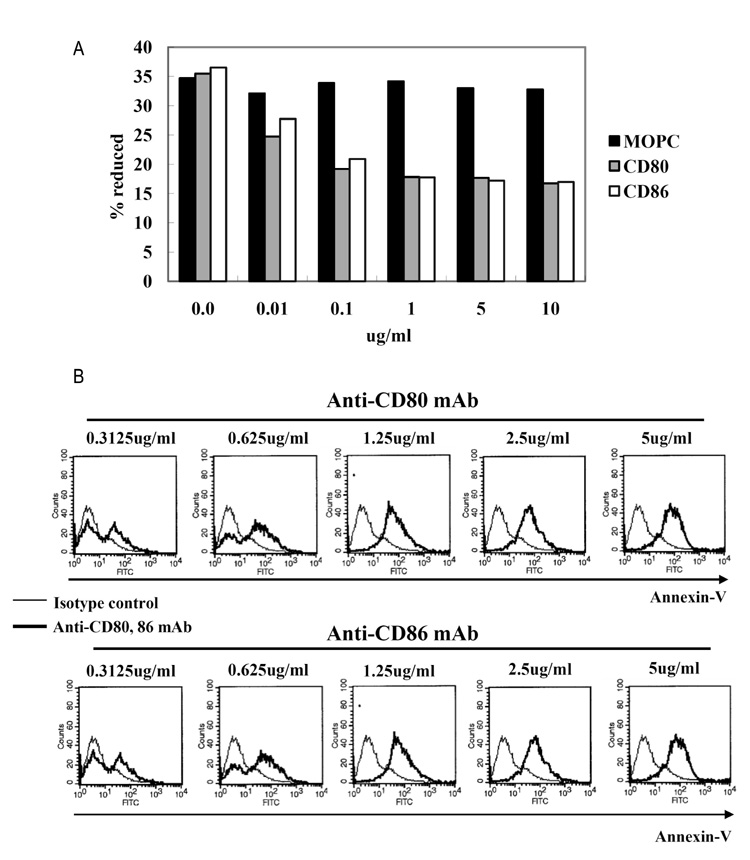
Cross-linking of CD80 and CD86 induced apoptosis and interfered with proliferation of EBV-transformed B cells, and dispersion of clumped cells. We also examined that their stimulation induced ROS accumulation and reduced CD54 expression. Interestingly, we observed that CD80 and CD86 diminished the expression of CD54 in different methods. Both CD80 and CD86 down-regulated activation of focal adhesion kinase. CD80 stimulus inhibited CD54 expression through mainly RhoA inactivation, while CD86 down-regulated Ras and JNK phosphorylation.
CONCLUSION
These results suggest that co-stimulatory CD80 and CD86 molecules, expressed EBV-transformed B cells, may play a role in apoptosis and cell adhesion.
MeSH Terms
Figure
Reference
-
1. Middeldorp JM, Brink AA, van den Brule AJ, Meijer CJ. Pathogenic roles for Epstein-Barr virus (EBV) gene products in EBV-associated proliferative disorders. Crit Rev Oncol Hematol. 2003. 45:1–36.
Article2. Thorley-Lawson DA, Babcock GJ. A model for persistent infection with Epstein-Barr virus: the stealth virus of human B cells. Life Sci. 1999. 65:1433–1453.
Article3. Dolcetti R, Masucci MG. Epstein-Barr virus: induction and control of cell transformation. J Cell Physiol. 2003. 196:207–218.
Article4. Comito MA, Sun Q, Lucas KG. Immunotherapy for Ep stein-Barr virus-associated tumors. Leuk Lymphoma. 2004. 45:1981–1987.5. Neitzel H. A routine method for the establishment of permanent growing lymphoblastoid cell lines. Hum Genet. 1986. 73:320–326.
Article6. Hur DY, Lee MH, Kim JW, Kim JH, Shin YK, Rho JK, Kwack KB, Lee WJ, Han BG. CD19 signalling improves the Epstein-Barr virus-induced immortalization of human B cell. Cell Prolif. 2005. 38:35–45.
Article7. Azuma M, Ito D, Yagita H, Okumura K, Phillips JH, Lanier LL, Somoza C. B70 antigen is a second ligand for CTLA-4 and CD28. Nature. 1993. 366:76–79.
Article8. Freeman GJ, Gribben JG, Boussiotis VA, Ng JW, Restivo VA Jr, Lombard LA, Gray GS, Nadler LM. Cloning of B7-2: a CTLA-4 counter-receptor that costimulates human T cell proliferation. Science. 1993. 262:909–911.
Article9. Larsen CP, Ritchie SC, Hendrix R, Linsley PS, Hathcock KS, Hodes RJ, Lowry RP, Pearson TC. Regulation of immunostimulatory function and costimulatory molecule (B7-1 and B7-2) expression on murine dendritic cells. J Immunol. 1994. 152:5208–5219.10. Zheng P, Wu Y, Guo Y, Lee C, Liu Y. B7-CTLA4 interaction enhances both production of antitumor cytotoxic T lymphocytes and resistance to tumor challenge. Proc Natl Acad Sci USA. 1998. 95:6284–6289.
Article11. Gordon J, Millsum MJ, Guy GR, Ledbetter JA. Resting B lymphocytes can be triggered directly through the CDw40 (Bp50) antigen. A comparison with IL-4-mediated signaling. J Immunol. 1988. 140:1425–1430.12. Goldstein MD, Watts TH. Identification of distinct domains in CD40 involved in B7-1 induction or growth inhibition. J Immunol. 1996. 157:2837–2843.13. Nakajima A, Kodama T, Morimoto S, Azuma M, Takeda K, Oshima H, Yoshino S, Yagita H, Okumura K. Antitumor effect of CD40 ligand: elicitation of local and systemic antitumor responses by IL-12 and B7. J Immunol. 1998. 161:1901–1907.14. Bergamo A, Bataille R, Pellat-Deceunynck C. CD40 and CD95 induce programmed cell death in the human myeloma cell line XG2. Br J Haematol. 1997. 97:652–655.
Article15. Mongini PK, Tolani S, Fattah RJ, Inman JK. Antigen receptor triggered upregulation of CD86 and CD80 in human B cells: augmenting role of the CD21/CD19 co-stimulatory complex and IL-4. Cell Immunol. 2002. 216:50–64.
Article16. Evans DE, Munks MW, Purkerson JM, Parker DC. Resting B lymphocytes as APC for naive T lymphocytes: dependence on CD40 ligand/CD40. J Immunol. 2000. 164:688–697.
Article17. Clatza A, Bonifaz LC, Vignali DA, Moreno J. CD40-induced aggregation of MHC class II and CD80 on the cell surface leads to an early enhancement in antigen presentation. J Immunol. 2003. 171:6478–6487.
Article18. Bluestone JA. New perspectives of CD28-B7-mediated T cell costimulation. Immunity. 1995. 2:555–559.
Article19. Jirapongsananuruk O, Hofer MF, Trumble AE, Norris DA, Leung DY. Enhanced expression of B7.2 (CD86) in patients with atopic dermatitis: a potential role in the modulation of IgE synthesis. J Immunol. 1998. 160:4622–4627.20. Ikemizu S, Gilbert RJ, Fennelly JA, Collins AV, Harlos K, Jones EY, Stuart DI, Davis SJ. Structure and dimerization of a soluble form of B7-1. Immunity. 2000. 12:51–60.
Article21. Bajorath J, Peach RJ, Linsley PS. Immunoglobulin fold characteristics of B7-1 (CD80) and B7-2 (CD86). Protein Sci. 1994. 3:2148–2150.
Article22. Heath AW, Chang R, Harada N, Santos-Argumedo L, Gordon J, Hannum C, Campbell D, Shanafelt AB, Clark EA, Torres R, Howard M. Antibodies to murine CD40 stimulate normal B cells but inhibit proliferation of B lymphoma cells. Cell Immunol. 1993. 152:468–480.
Article23. Borriello F, Sethna MP, Boyd SD, Schweitzer AN, Tivol EA, Jacoby D, Strom TB, Simpson EM, Freeman GJ, Sharpe AH. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997. 6:303–313.
Article24. Suvas S, Singh V, Sahdev S, Vohra H, Agrewala JN. Distinct role of CD80 and CD86 in the regulation of the activation of B cell and B cell lymphoma. J Biol Chem. 2002. 277:7766–7775.
Article25. Lukas Z, Dvorak K. Review article adhesion molecules in biology and oncology. Acta Vet Brno. 2004. 73:93–104.26. Roebuck KA, Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J Leukoc Biol. 1999. 66:876–888.
Article27. Rincon J, Prieto J, Patarroyo M. Expression of integrins and other adhesion molecules in Epstein-Barr virus-transformed B lymphoblastoid cells and Burkitt's lymphoma cells. Int J Cancer. 1992. 51:452–458.
Article28. Mehl AM, Floettmann JE, Jones M, Brennan P, Rowe M. Characterization of intercellular adhesion molecule-1 regulation by Epstein-Barr virus-encoded latent membrane protein-1 identifies pathways that cooperate with nuclear factor kappa B to activate transcription. J Biol Chem. 2001. 276:984–992.
Article29. Kosco MH, Pflugfelder E, Gray D. Follicular dendritic cell-dependent adhesion and proliferation of B cells in vitro. J Immunol. 1992. 148:2331–2339.30. Koopman G, Keehnen RM, Lindhout E, Newman W, Shimizu Y, van Seventer GA, de Groot C, Pals ST. Adhesion through the LFA-1 (CD11a/CD18)-ICAM-1 (CD54) and the VLA-4 (CD49d)-VCAM-1 (CD106) pathways prevents apoptosis of germinal center B cells. J Immunol. 1994. 152:3760–3767.31. Gonzalo JA, Martinez C, Springer TA, Gutierrez-Ramos JC. ICAM-1 is required for T cell proliferation but not for anergy or apoptosis induced by Staphylococcus aureus enterotoxin B in vivo. Int Immunol. 1995. 7:1691–1698.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- p190RhoGAP and Rap-dependent RhoGAP (ARAP3) inactivate RhoA in response to nerve growth factor leading to neurite outgrowth from PC12 cells
- Enhancement of Proliferation and Antigen Presentation of Human B Cells in Vitro by K562 Cells Expressing CD40L
- Vitamin C Up-regulates Expression of CD80, CD86 and MHC Class II on Dendritic Cell Line, DC-1 Via the Activation of p38 MAPK
- Antisense GLUT1 RNA suppresses the transforming phenotypes of NIH 3T3 cells transformed by N-Ras
- The Change of Immunoactivity of Dendritic Cells Induced by Mouse 4-1BBL Recombinant Adenovirus