J Vet Sci.
2017 Sep;18(3):387-397. 10.4142/jvs.2017.18.3.387.
Gintonin, an exogenous ginseng-derived LPA receptor ligand, promotes corneal wound healing
- Affiliations
-
- 1Ginsentology Research Laboratory and Department of Physiology, College of Veterinary Medicine, Konkuk University, Seoul 05029, Korea. synah@konkuk.ac.kr
- 2Veterinary Medical Teaching Hospital, Konkuk University, Seoul 05029, Korea.
- 3Center for Neuroscience, Korea Institute of Science and Technology, Seoul 02792, Korea.
- 4Neuropsychopharmacology and toxicology program, College of Pharmacy, Kangwon National University, Chuncheon 24341, Korea.
- 5Department of Convergence Medical Science, College of Oriental Medicine, Kyung Hee University, Seoul 02447, Korea.
- KMID: 2412457
- DOI: http://doi.org/10.4142/jvs.2017.18.3.387
Abstract
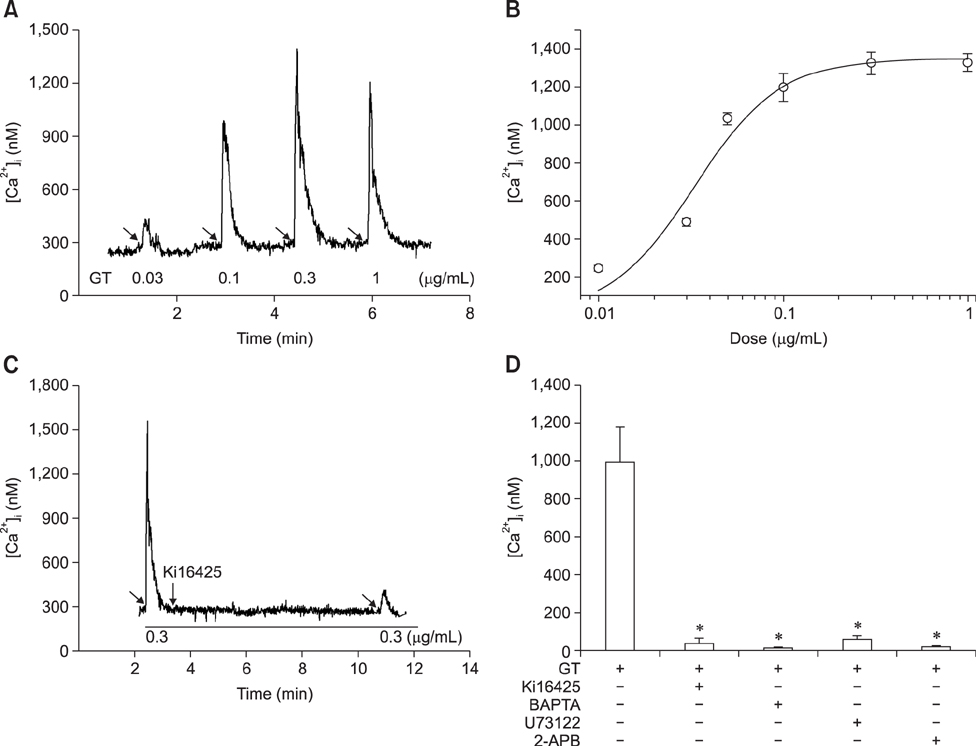
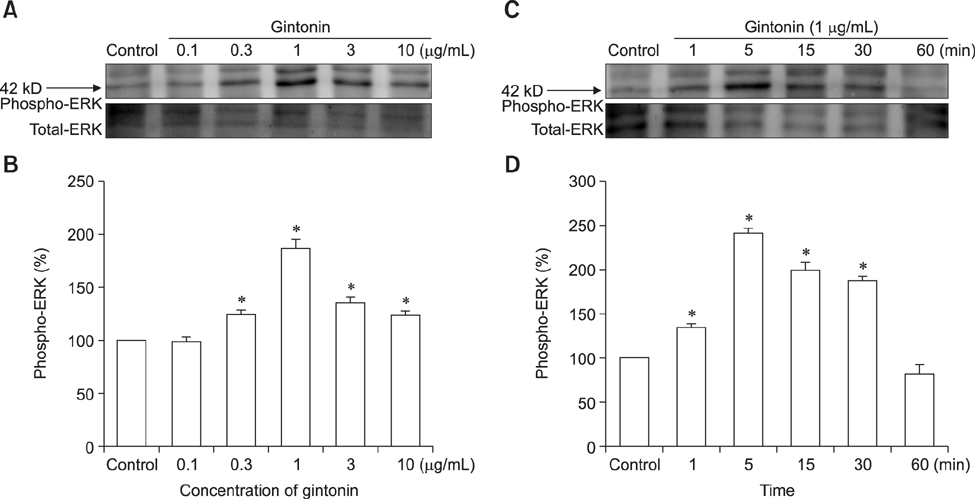
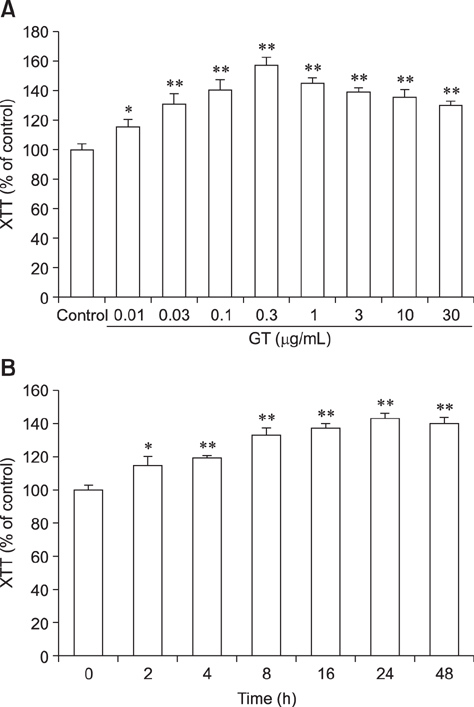
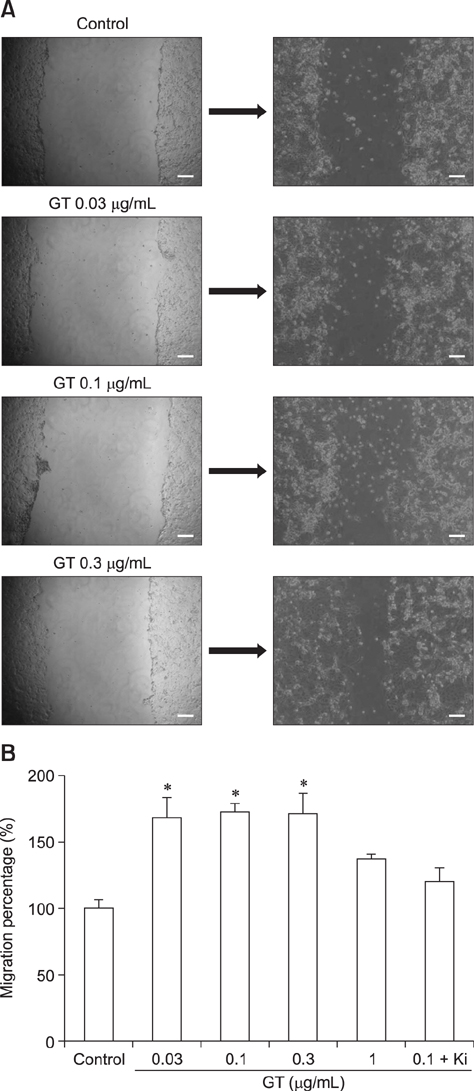
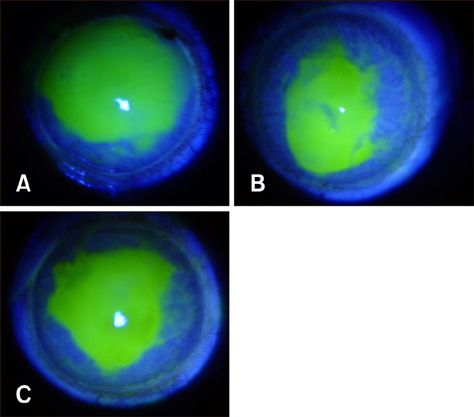
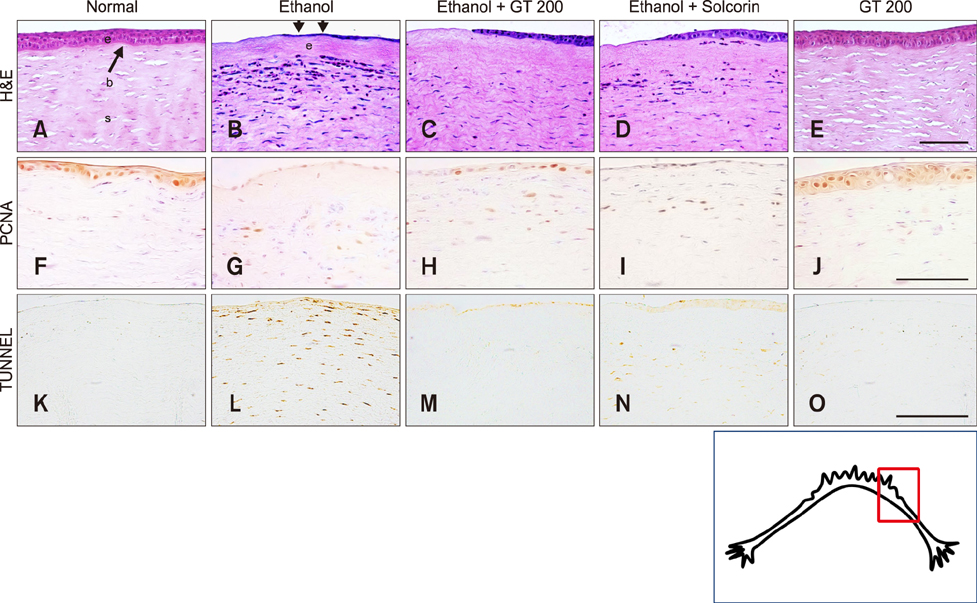
- Ginseng gintonin is an exogenous ligand of lysophosphatidic acid (LPA) receptors. Accumulating evidence shows LPA helps in rapid recovery of corneal damage. The aim of this study was to evaluate the therapeutic efficacy of gintonin in a rabbit model of corneal damage. We investigated the signal transduction pathway of gintonin in human corneal epithelium (HCE) cells to elucidate the underlying molecular mechanism. We next evaluated the therapeutic effects of gintonin, using a rabbit model of corneal damage, by undertaking histochemical analysis. Treatment of gintonin to HCE cells induced transient increases of [Ca²âº](i) in concentration-dependent and reversible manners. Gintonin-mediated mobilization of [Ca²âº](i) was attenuated by LPA1/3 receptor antagonist Ki16425, phospholipase C inhibitor U73122, inositol 1,4,5-triphosphate receptor antagonist 2-APB, and intracellular Ca²âº chelator BAPTA-AM. Gintonin facilitated in vitro wound healing in a concentration-dependent manner. When applied as an eye-drop to rabbits with corneal damage, gintonin rapidly promoted recovery. Histochemical analysis showed gintonin decreased corneal apoptosis and increased corneal cell proliferation. We demonstrated that LPA receptor activation by gintonin is linked to in vitro and in vivo therapeutic effects against corneal damage. Gintonin can be applied as a clinical agent for the rapid healing of corneal damage.
Keyword
MeSH Terms
-
Animals
Blotting, Western/veterinary
Calcium/metabolism
Cells, Cultured
Cornea/drug effects/pathology
Corneal Injuries/*drug therapy/pathology
Dose-Response Relationship, Drug
Humans
Male
Plant Extracts/*therapeutic use
Rabbits
Receptors, Lysophosphatidic Acid/drug effects
Wound Healing/*drug effects
Plant Extracts
Receptors, Lysophosphatidic Acid
Calcium
Figure
Reference
-
1. Araki-Sasaki K, Aizawa S, Hiramoto M, Nakamura M, Iwase O, Nakata K, Sasaki Y, Mano T, Handa H, Tano Y. Substance P-induced cadherin expression and its signal transduction in a cloned human corneal epithelial cell line. J Cell Physiol. 2000; 182:189–195.
Article2. Araki-Sasaki K, Ohashi Y, Sasabe T, Hayashi K, Watanabe H, Tano Y, Handa H. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci. 1995; 36:614–621.3. Cho IH, Hong J, Suh EC, Kim JH, Lee H, Lee JE, Lee S, Kim CH, Kim DW, Jo EK, Lee KE, Karin M, Lee SJ. Role of microglial IKKβ in kainic acid-induced hippocampal neuronal cell death. Brain. 2008; 131:3019–3033.
Article4. Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T, Lin ME, Teo ST, Park KE, Mosley AN, Chun J. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol. 2010; 50:157–186.
Article5. Choi SH, Hong MK, Kim HJ, Ryoo N, Rhim H, Nah SY, Kang LW. Structure of ginseng major latex-like protein 151 and its proposed lysophosphatidic acid-binding mechanism. Acta Crystallogr D Biol Crystallogr. 2015; 71:1039–1050.
Article6. Choi SH, Kim HJ, Kim BR, Shin TJ, Hwang SH, Lee BH, Lee SM, Rhim H, Nah SY. Gintonin, a ginseng-derived lysophosphatidic acid receptor ligand, potentiates ATPgated P2X1 receptor channel currents. Mol Cells. 2013; 35:142–150.
Article7. Choi SH, Shin TJ, Lee BH, Hwang SH, Kang J, Kim HJ, Park CW, Nah SY. An edible gintonin preparation from ginseng. J Ginseng Res. 2011; 35:471–478.
Article8. Dawson DG, Edelhauser HF, Grossniklaus HE. Long-term histopathologic findings in human corneal wounds after refractive surgical procedures. Am J Ophthalmol. 2005; 139:168–178.
Article9. Hwang SH, Lee BH, Choi SH, Kim HJ, Jung SW, Kim HS, Shin HC, Park HJ, Park KH, Lee MK, Nah SY. Gintonin, a novel ginseng-derived lysophosphatidic acid receptor ligand, stimulates neurotransmitter release. Neurosci Lett. 2015; 584:356–361.
Article10. Hwang SH, Lee BH, Kim HJ, Cho HJ, Shin HC, Im KS, Choi SH, Shin TJ, Lee SM, Nam SW, Kim HC, Rhim H, Nah SY. Suppression of metastasis of intravenously-inoculated B16/F10 melanoma cells by the novel ginseng-derived ingredient, gintonin: involvement of autotaxin inhibition. Int J Oncol. 2013; 42:317–326.
Article11. Hwang SH, Shin EJ, Shin TJ, Lee BH, Choi SH, Kang JY, Kim HJ, Kwon SH, Jang CG, Lee JH, Kim HC, Nah SY. Gintonin, a ginseng-derived lysophosphatidic acid receptor ligand, attenuates Alzheimer's disease-related neuropathies: involvement of non-amyloidogenic processing. J Alzheimers Dis. 2012; 31:207–223.
Article12. Hwang SH, Shin TJ, Choi SH, Cho HJ, Lee BH, Pyo MK, Lee JH, Kang J, Kim HJ, Park CW, Shin HC, Nah SY. Gintonin, newly identified compounds from ginseng, is novel lysophosphatidic acids-protein complexes and activates G protein-coupled lysophosphatidic acid receptors with high affinity. Mol Cells. 2012; 33:151–162.
Article13. Im DS, Nah SY. Yin and Yang of ginseng pharmacology: ginsenosides vs gintonin. Acta Pharmacol Sin. 2013; 34:1367–1373.
Article14. Jang M, Lee MJ, Cho IH. Ethyl pyruvate ameliorates 3-nitropropionic acid-induced striatal toxicity through anti-neuronal cell death and anti-inflammatory mechanisms. Brain Behav Immun. 2014; 38:151–165.
Article15. Kim JY, Choi YM, Jeong SW, Williams DL. Effect of bovine freeze-dried amniotic membrane (Amnisite-BA) on uncomplicated canine corneal erosion. Vet Ophthalmol. 2009; 12:36–42.
Article16. Kim YM, Namkoong S, Yun YG, Hong HD, Lee YC, Ha KS, Lee H, Kwon HJ, Kwon YG, Kim YM. Water extract of Korean red ginseng stimulates angiogenesis by activating the PI3K/Akt-dependent ERK1/2 and eNOS pathways in human umbilical vein endothelial cells. Biol Pharm Bull. 2007; 30:1674–1679.
Article17. Kimura Y, Sumiyoshi M, Kawahira K, Sakanaka M. Effects of ginseng saponins isolated from Red Ginseng roots on burn wound healing in mice. Br J Pharmacol. 2006; 148:860–870.
Article18. Kuo IC. Corneal wound healing. Curr Opin Ophthalmol. 2004; 15:311–315.
Article19. Lee MJ, Jang M, Choi J, Lee G, Min HJ, Chung WS, Kim JI, Jee Y, Chae Y, Kim SH, Lee SJ, Cho IH. Bee venom acupuncture alleviates experimental autoimmune encephalomyelitis by upregulating regulatory T cells and suppressing Th1 and Th17 responses. Mol Neurobiol. 2016; 53:1419–1445.
Article20. Liliom K, Guan Z, Tseng JL, Desiderio DM, Tigyi G, Watsky MA. Growth factor-like phospholipids generated after corneal injury. Am J Physiol. 1998; 274:C1065–C1074.21. Lu L, Reinach PS, Kao WW. Corneal epithelial wound healing. Exp Biol Med (Maywood). 2001; 226:653–664.
Article22. Nah SY. Ginseng ginsenoside pharmacology in the nervous system: involvement in the regulation of ion channels and receptors. Front Physiol. 2014; 5:98.
Article23. Pazyar N, Yaghoobi R, Rafiee E, Mehrabian A, Feily A. Skin wound healing and phytomedicine: a review. Skin Pharmacol Physiol. 2014; 27:303–310.
Article24. Pyo MK, Choi SH, Shin TJ, Hwang SH, Lee BH, Kang J, Kim HJ, Lee SH, Nah SY. A simple method for the preparation of crude gintonin from ginseng root, stem, and leaf. J Ginseng Res. 2011; 35:209–218.
Article25. Shin TJ, Kim HJ, Kwon BJ, Choi SH, Kim HB, Hwang SH, Lee BH, Lee SM, Zukin RS, Park JH, Kim HC, Rhim H, Lee JH, Nah SY. Gintonin, a ginseng-derived novel ingredient, evokes long-term potentiation through N-methyl-D-aspartic acid receptor activation: involvement of LPA receptors. Mol Cells. 2012; 34:563–572.
Article26. Tanaka T, Horiuchi G, Matsuoka M, Hirano K, Tokumura A, Koike T, Satouchi K. Formation of lysophosphatidic acid, a wound-healing lipid, during digestion of cabbage leaves. Biosci Biotechnol Biochem. 2009; 73:1293–1300.
Article27. Wang DA, Du H, Jaggar JH, Brindley DN, Tigyi GJ, Watsky MA. Injury-elicited differential transcriptional regulation of phospholipid growth factor receptors in the cornea. Am J Physiol Cell Physiol. 2002; 283:C1646–C1654.
Article28. Watsky MA, Griffith M, Wang DA, Tigyi GJ. Phospholipid growth factors and corneal wound healing. Ann N Y Acad Sci. 2000; 905:142–158.
Article29. Watterson KR, Lanning DA, Diegelmann RF, Spiegel S. Regulation of fibroblast functions by lysophospholipid mediators: potential roles in wound healing. Wound Repair Regen. 2007; 15:607–616.
Article30. Xu KP, Yin J, Yu FS. Lysophosphatidic acid promoting corneal epithelial wound healing by transactivation of epidermal growth factor receptor. Invest Ophthalmol Vis Sci. 2007; 48:636–643.
Article31. Yung YC, Stoddard NC, Chun J. LPA receptor signaling: pharmacology, physiology, and pathophysiology. J Lipid Res. 2014; 55:1192–1214.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gintonin facilitates catecholamine secretion from the perfused adrenal medulla
- Effects of 0.1% Dexamethasone on Experimental corneal Epithelial Healing Following Alkali Wounds
- Corneal Epithelial Wound Healing After Excimer Laser Photorefractive Keratectomy
- Basic Study on the Effect of Korean Ginseng upon Fracture Healing of the Bone
- The Effect of Epidermal Growth Factor on Cornea Epithelial Wound Healing in Excimer Laser Keratectomized Rabbits