Cancer Res Treat.
2018 Apr;50(2):495-505. 10.4143/crt.2016.577.
ALK Protein Expression Is Related to Neuroblastoma Aggressiveness But Is Not Independent Prognostic Factor
- Affiliations
-
- 1Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Department of Pathology, Seoul National University College of Medicine, Seoul, Korea. shparknp@snu.ac.kr
- 3Department of Pediatrics, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea. hyshin@snu.ac.kr
- KMID: 2411138
- DOI: http://doi.org/10.4143/crt.2016.577
Abstract
- PURPOSE
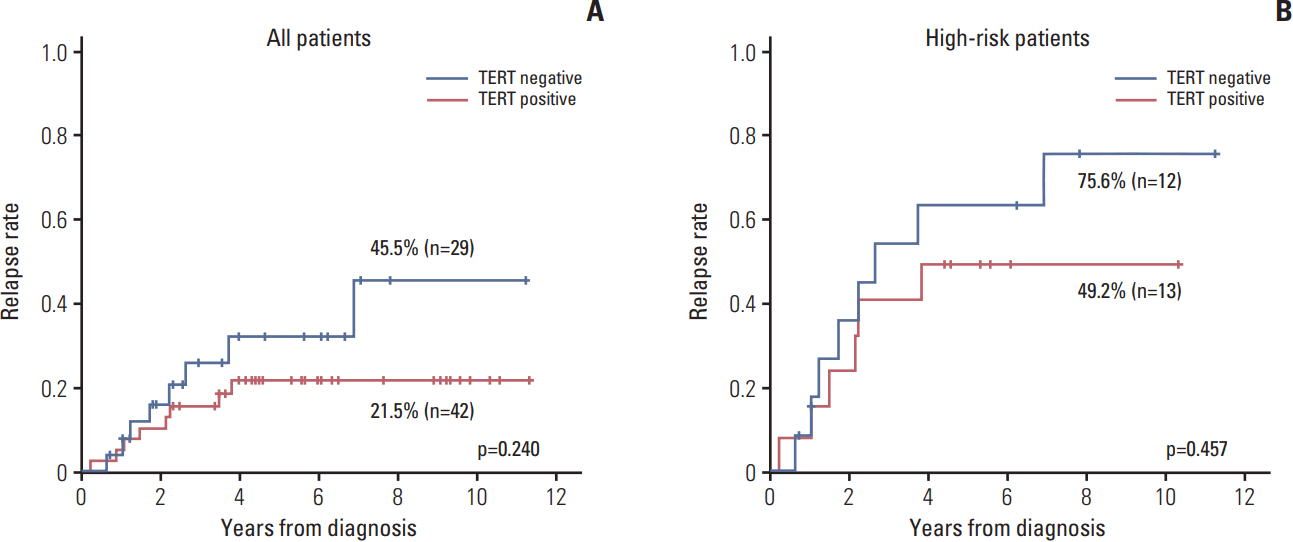
In this study, anaplastic lymphoma kinase (ALK) mutation and amplification, ALK protein expression, loss of the nuclear alpha thalassemia/mental retardation syndrome X-linked (ATRX) protein, and telomerase reverse transcriptase (TERT) protein expressionwere studied to investigate potential correlations between these molecular characteristics and clinical features or outcomes in neuroblastoma.
MATERIALS AND METHODS
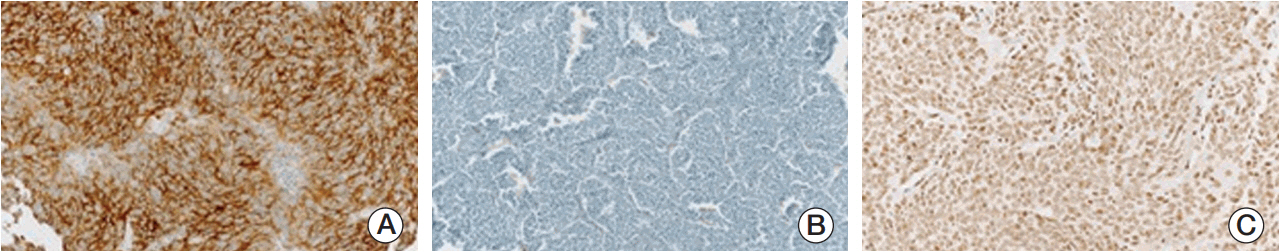
Seventy-two patients were enrolled in this study. Polymerase chain reaction amplification and direct sequencing were used for mutation analysis. ALK and MYCN amplifications were detected by fluorescence in situ hybridization. Protein expressionwas evaluated by immunohistochemical (IHC) staining.
RESULTS
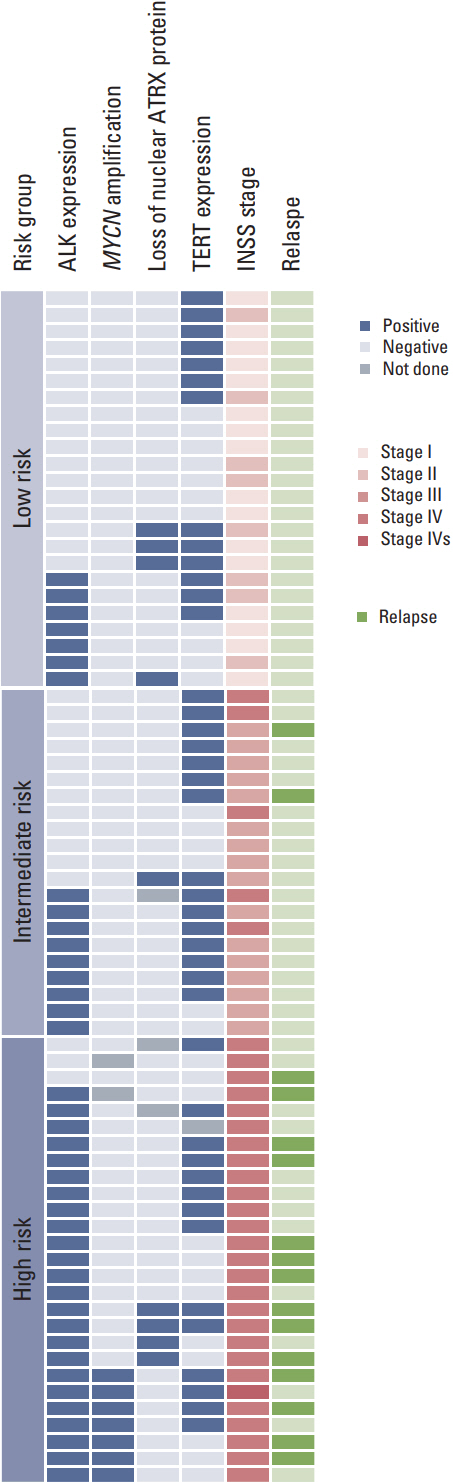
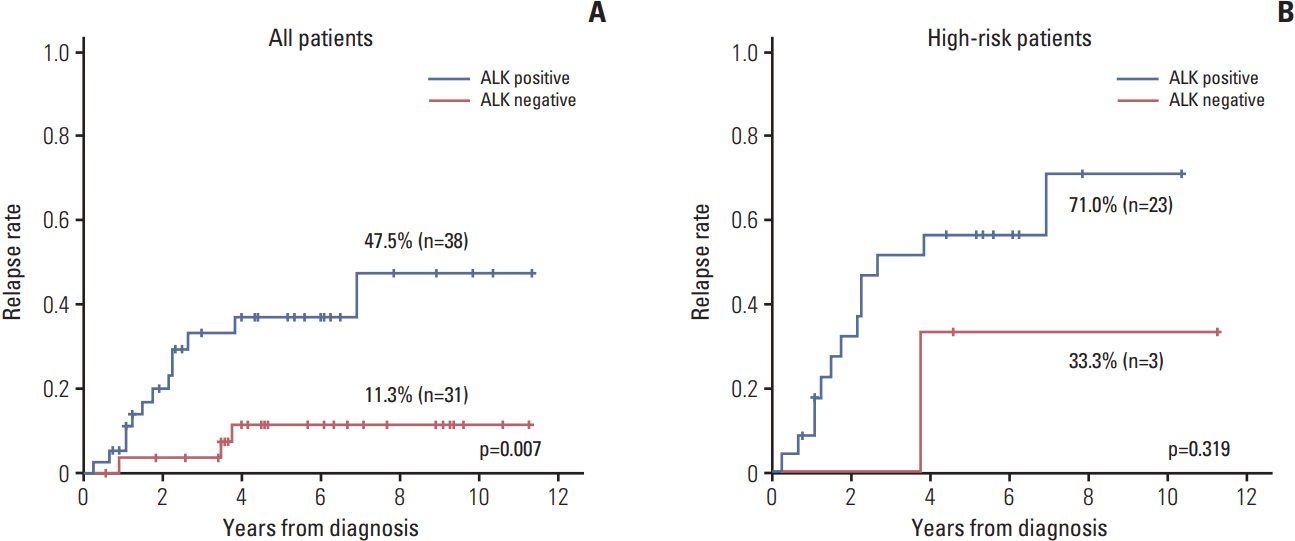
ALK mutation was found in only two patients (4.1%); ALK amplification was not detected. ALK positivity, loss of nuclear ATRX protein, TERT positivity by IHC were detected in 40 (55.6%), nine (13.0%), and 42 (59.2%) patients, respectively. The incidence of ALK expression increased in accordance with increasing tumor stage (p=0.001) and risk group (p < 0.001). The relapse rate was significantly higher in ALK+ patients compared to that of other patients (47.5% vs. 11.3%, p=0.007). However, there was no significant difference in relapse rate when the survival analysis was confined to the high-risk patients.
CONCLUSION
Although ALK mutation was rare and no amplification was observed, ALK protein expression was found in a significant number of patients and was correlated with advanced stage and high-risk neuroblastoma. ALK protein expression could be considered as a marker related to the aggressive neuroblastoma, but it was not the independent prognostic factor for the outcome.
MeSH Terms
Figure
Reference
-
References
1. Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007; 369:2106–20.
Article2. Ambros PF, Ambros IM, Brodeur GM, Haber M, Khan J, Nakagawara A, et al. International consensus for neuroblastoma molecular diagnostics: report from the International Neuroblastoma Risk Group (INRG) Biology Committee. Br J Cancer. 2009; 100:1471–82.
Article3. Janoueix-Lerosey I, Schleiermacher G, Michels E, Mosseri V, Ribeiro A, Lequin D, et al. Overall genomic pattern is a predictor of outcome in neuroblastoma. J Clin Oncol. 2009; 27:1026–33.
Article4. Ladenstein R, Ambros IM, Potschger U, Amann G, Urban C, Fink FM, et al. Prognostic significance of DNA di-tetraploidy in neuroblastoma. Med Pediatr Oncol. 2001; 36:83–92.
Article5. Stallings RL. Are chromosomal imbalances important in cancer? Trends Genet. 2007; 23:278–83.
Article6. Schleiermacher G, Mosseri V, London WB, Maris JM, Brodeur GM, Attiyeh E, et al. Segmental chromosomal alterations have prognostic impact in neuroblastoma: a report from the INRG project. Br J Cancer. 2012; 107:1418–22.
Article7. Chen Y, Takita J, Choi YL, Kato M, Ohira M, Sanada M, et al. Oncogenic mutations of ALK kinase in neuroblastoma. Nature. 2008; 455:971–4.
Article8. George RE, Sanda T, Hanna M, Frohling S, Luther W 2nd, Zhang J, et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature. 2008; 455:975–8.
Article9. Janoueix-Lerosey I, Lequin D, Brugieres L, Ribeiro A, de Pontual L, Combaret V, et al. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature. 2008; 455:967–70.
Article10. Mosse YP, Laudenslager M, Longo L, Cole KA, Wood A, Attiyeh EF, et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature. 2008; 455:930–5.
Article11. Miyake I, Hakomori Y, Shinohara A, Gamou T, Saito M, Iwamatsu A, et al. Activation of anaplastic lymphoma kinase is responsible for hyperphosphorylation of ShcC in neuroblastoma cell lines. Oncogene. 2002; 21:5823–34.
Article12. Osajima-Hakomori Y, Miyake I, Ohira M, Nakagawara A, Nakagawa A, Sakai R. Biological role of anaplastic lymphoma kinase in neuroblastoma. Am J Pathol. 2005; 167:213–22.
Article13. Blackburn EH. Telomeres and telomerase: the means to the end (Nobel lecture). Angew Chem Int Ed Engl. 2010; 49:7405–21.
Article14. Cheung NK, Zhang J, Lu C, Parker M, Bahrami A, Tickoo SK, et al. Association of age at diagnosis and genetic mutations in patients with neuroblastoma. JAMA. 2012; 307:1062–71.
Article15. Peifer M, Hertwig F, Roels F, Dreidax D, Gartlgruber M, Menon R, et al. Telomerase activation by genomic rearrangements in high-risk neuroblastoma. Nature. 2015; 526:700–4.
Article16. Valentijn LJ, Koster J, Zwijnenburg DA, Hasselt NE, van Sluis P, Volckmann R, et al. TERT rearrangements are frequent in neuroblastoma and identify aggressive tumors. Nat Genet. 2015; 47:1411–4.
Article17. Maris JM. The biologic basis for neuroblastoma heterogeneity and risk stratification. Curr Opin Pediatr. 2005; 17:7–13.
Article18. Subramaniam MM, Piqueras M, Navarro S, Noguera R. Aberrant copy numbers of ALK gene is a frequent genetic alteration in neuroblastomas. Hum Pathol. 2009; 40:1638–42.
Article19. Bresler SC, Wood AC, Haglund EA, Courtright J, Belcastro LT, Plegaria JS, et al. Differential inhibitor sensitivity of anaplastic lymphoma kinase variants found in neuroblastoma. Sci Transl Med. 2011; 3:108ra14.
Article20. Infarinato NR, Park JH, Krytska K, Ryles HT, Sano R, Szigety KM, et al. The ALK/ROS1 inhibitor PF-06463922 overcomes primary resistance to crizotinib in ALK-driven neuroblastoma. Cancer Discov. 2016; 6:96–107.
Article21. Palmer RH, Vernersson E, Grabbe C, Hallberg B. Anaplastic lymphoma kinase: signalling in development and disease. Biochem J. 2009; 420:345–61.
Article22. Bonvini P, Zin A, Alaggio R, Pawel B, Bisogno G, Rosolen A. High ALK mRNA expression has a negative prognostic significance in rhabdomyosarcoma. Br J Cancer. 2013; 109:3084–91.
Article23. Gasparini P, Casanova M, Villa R, Collini P, Alaggio R, Zin A, et al. Anaplastic lymphoma kinase aberrations correlate with metastatic features in pediatric rhabdomyosarcoma. Oncotarget. 2016; 7:58903–14.
Article24. Passoni L, Longo L, Collini P, Coluccia AM, Bozzi F, Podda M, et al. Mutation-independent anaplastic lymphoma kinase overexpression in poor prognosis neuroblastoma patients. Cancer Res. 2009; 69:7338–46.
Article25. Schulte JH, Bachmann HS, Brockmeyer B, Depreter K, Oberthur A, Ackermann S, et al. High ALK receptor tyrosine kinase expression supersedes ALK mutation as a determining factor of an unfavorable phenotype in primary neuroblastoma. Clin Cancer Res. 2011; 17:5082–92.26. Duijkers FA, Gaal J, Meijerink JP, Admiraal P, Pieters R, de Krijger RR, et al. High anaplastic lymphoma kinase immunohistochemical staining in neuroblastoma and ganglioneuroblastoma is an independent predictor of poor outcome. Am J Pathol. 2012; 180:1223–31.
Article27. Schonherr C, Ruuth K, Kamaraj S, Wang CL, Yang HL, Combaret V, et al. Anaplastic lymphoma kinase (ALK) regulates initiation of transcription of MYCN in neuroblastoma cells. Oncogene. 2012; 31:5193–200.
Article28. Umapathy G, El Wakil A, Witek B, Chesler L, Danielson L, Deng X, et al. The kinase ALK stimulates the kinase ERK5 to promote the expression of the oncogene MYCN in neuroblastoma. Sci Signal. 2014; 7:ra102.
Article29. Ohali A, Avigad S, Ash S, Goshen Y, Luria D, Feinmesser M, et al. Telomere length is a prognostic factor in neuroblastoma. Cancer. 2006; 107:1391–9.
Article30. Lovejoy CA, Li W, Reisenweber S, Thongthip S, Bruno J, de Lange T, et al. Loss of ATRX, genome instability, and an altered DNA damage response are hallmarks of the alternative lengthening of telomeres pathway. PLoS Genet. 2012; 8:e1002772.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Alteration of Alk in Neuroblastoma
- Immunohistochemical expression of anaplastic lymphoma kinase in neuroblastoma and its relations with some clinical and histopathological features
- Prognostic role of ALK-1 and h-TERT expression in glioblastoma multiforme: correlation with ALK gene alterations
- Clinicopathological Analysis of Systemic Anaplastic Large Cell Lymphoma
- Correlation between Expression of CD44, Bcl-2, PCNA, and Apoptosis in Neuroblastoma According to Shimada Histology