J Pathol Transl Med.
2021 May;55(3):212-224. 10.4132/jptm.2021.03.15.
Prognostic role of ALK-1 and h-TERT expression in glioblastoma multiforme: correlation with ALK gene alterations
- Affiliations
-
- 1Department of Pathology, Faculty of Medicine, Assiut University, Assiut, Egypt
- 2Department of Clinical Pathology, South Egypt Cancer Institute, Assiut University, Assiut, Egypt
- 3Department of Radiation Oncology, South Egypt Cancer Institute, Assiut University, Assiut, Egypt
- 4Department of Medical Oncology, South Egypt Cancer Institute, Assiut University, Assiut, Egypt
- 5Department of Oncologic Pathology, South Egypt Cancer Institute, Assiut University, Assiut, Egypt
- KMID: 2515926
- DOI: http://doi.org/10.4132/jptm.2021.03.15
Abstract
- Background
Anaplastic lymphoma kinase (ALK) is a receptor tyrosine kinase that is expressed in the developing central and peripheral nervous systems during embryogenesis. Human telomerase reverse transcriptase (h-TERT) protein resumption is the main process of preservation of telomeres that maintains DNA integrity. The present study aims to evaluate the prognostic role of ALK-1 and h-TERT protein expression and their correlation with ALK gene alterations in glioblastoma multiforme (GBM).
Methods
The current study is a retrospective study on a cohort of patients with GBM (n = 53) that attempted to detect ALK gene alterations using fluorescence in situ hybridization. ALK-1 and h-TERT proteins were evaluated using immunohistochemistry.
Results
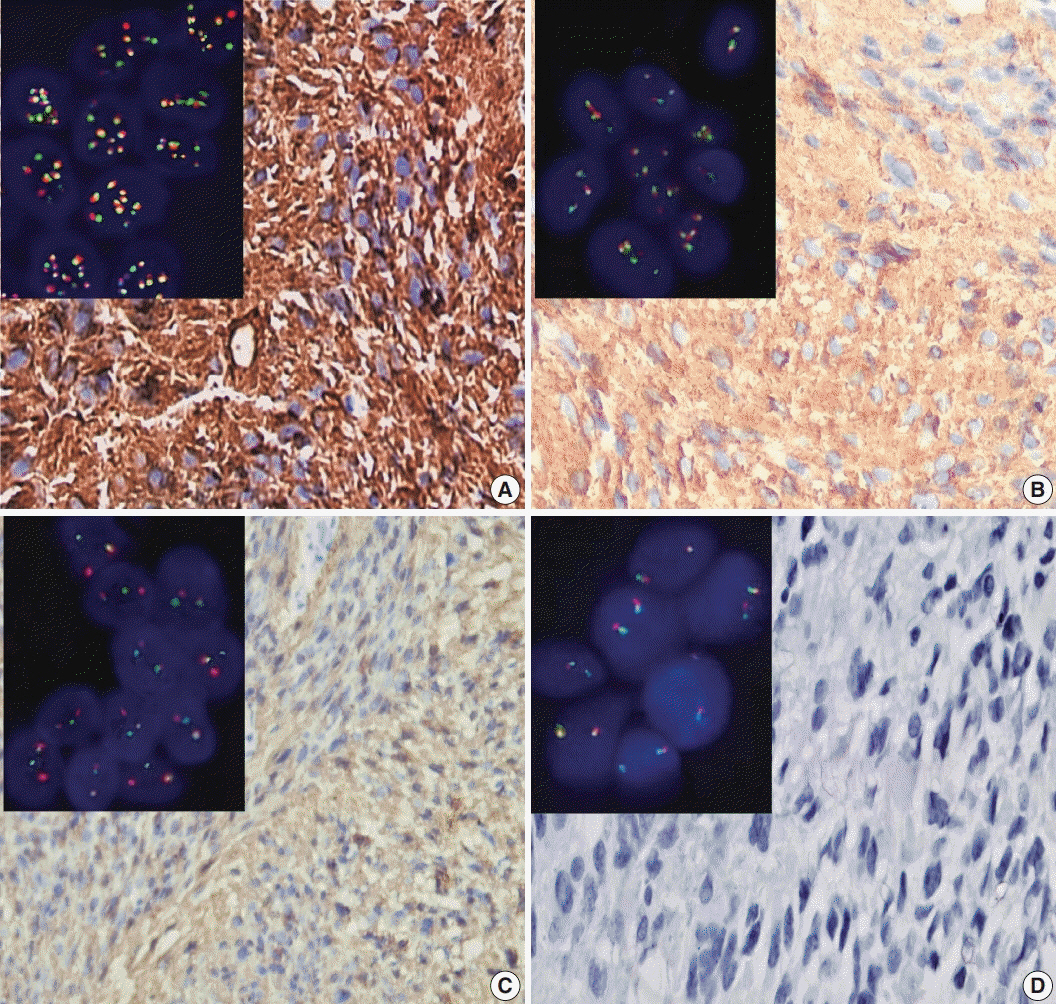
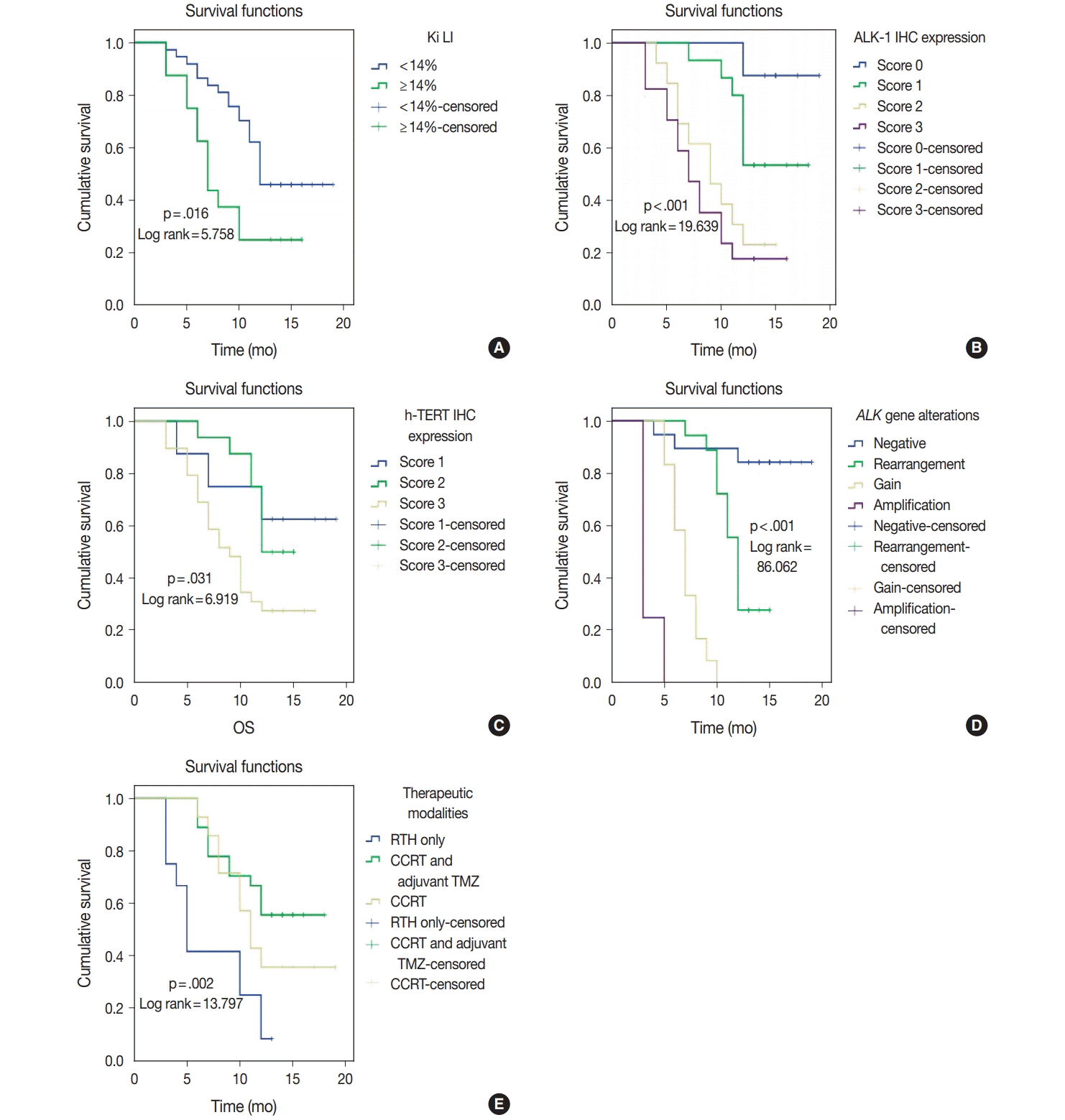
Score 3 ALK-1 expression was significantly associated with male sex, tumor multiplicity, Ki labeling index (Ki LI), and type of therapeutic modality. Score 3 h-TERT expression exhibited a significant association with Ki LI. ALK gene amplifications (ALK-A) were significantly associated with increased Ki LI and therapeutic modalities. Score 3 ALK-1 protein expression, score 3 h-TERT protein expression, and ALK-A were associated with poor overall survival (OS) and progression-free survival (PFS). Multivariate analysis for OS revealed that ALK gene alterations were an independent prognostic factor for OS and PFS.
Conclusions
High protein expression of both ALK-1 and h-TERT, as well as ALK-A had a poor impact on the prognosis of GBM. Further studies are needed to establish the underlying mechanisms.
Keyword
Figure
Reference
-
References
1. Stoyanov GS, Dzhenkov D, Ghenev P, Iliev B, Enchev Y, Tonchev AB. Cell biology of glioblastoma multiforme: from basic science to diagnosis and treatment. Med Oncol. 2018; 35:27.
Article2. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016; 131:803–20.
Article3. Chiarle R, Voena C, Ambrogio C, Piva R, Inghirami G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer. 2008; 8:11–23.
Article4. Chiba R, Akiya M, Hashimura M, et al. ALK signaling cascade confers multiple advantages to glioblastoma cells through neovascularization and cell proliferation. PLoS One. 2017; 12:e0183516.
Article5. Karagkounis G, Stranjalis G, Argyrakos T, et al. Anaplastic lymphoma kinase expression and gene alterations in glioblastoma: correlations with clinical outcome. J Clin Pathol. 2017; 70:593–9.
Article6. Wojas-Krawczyk K, Krawczyk PA, Ramlau RA, et al. The analysis of ALK gene rearrangement by fluorescence in situ hybridization in non-small cell lung cancer patients. Contemp Oncol (Pozn). 2013; 17:484–92.7. Zito Marino F, Botti G, Aquino G, et al. Unproductive effects of ALK gene amplification and copy number gain in non-small-cell lung cancer: ALK gene amplification and copy gain in NSCLC. Int J Mol Sci. 2020; 21:4927.8. Hafezi F, Perez Bercoff D. The solo play of TERT promoter mutations. Cells. 2020; 9:749.9. Leao R, Apolonio JD, Lee D, Figueiredo A, Tabori U, Castelo-Branco P. Mechanisms of human telomerase reverse transcriptase (hTERT) regulation: clinical impacts in cancer. J Biomed Sci. 2018; 25:22.
Article10. Potharaju M, Mathavan A, Mangaleswaran B, et al. Clinicopathological analysis of HIF-1alpha and TERT on survival outcome in glioblastoma patients: a prospective, single institution study. J Cancer. 2019; 10:2397–406.
Article11. Persson A, Englund E. Different assessments of immunohistochemically stained Ki-67 and hTERT in glioblastoma multiforme yield variable results: a study with reference to survival prognosis. Clin Neuropathol. 2008; 27:224–33.
Article12. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352:987–96.
Article13. Le Rhun E, Chamberlain MC, Zairi F, et al. Patterns of response to crizotinib in recurrent glioblastoma according to ALK and MET molecular profile in two patients. CNS Oncol. 2015; 4:381–6.
Article14. Alidousty C, Duerbaum N, Wagener-Ryczek S, et al. Prevalence and potential biological role of TERT amplifications in ALK translocated adenocarcinoma of the lung. Histopathology. 2021; 78:578–85.15. Saha R, Chatterjee U, Mandal S, Saha K, Chatterjee S, Ghosh SN. Expression of phosphatase and tensin homolog, epidermal growth factor receptor, and Ki-67 in astrocytoma: a prospective study in a tertiary care hospital. Indian J Med Paediatr Oncol. 2014; 35:149–55.
Article16. McLeer-Florin A, Moro-Sibilot D, Melis A, et al. Dual IHC and FISH testing for ALK gene rearrangement in lung adenocarcinomas in a routine practice: a French study. J Thorac Oncol. 2012; 7:348–54.17. Salido M, Pijuan L, Martinez-Aviles L, et al. Increased ALK gene copy number and amplification are frequent in non-small cell lung cancer. J Thorac Oncol. 2011; 6:21–7.
Article18. Hudson L, Kulig K, Young D, McLendon R, Abemethy A. ALK and cMET expression in glioblastoma multiforme: implications for therapeutic targeting. Mol Cancer Ther. 2011; 10(11 Suppl):A42.19. Kulig K, McLendon RE, Locke SC, et al. MET and ALK in glioblastoma multiforme (GBM): comparison of IHC and FISH. J Clin Oncol. 2012; 30(15 Suppl):2021.
Article20. Peretti U, Ferrara R, Pilotto S, et al. ALK gene copy number gains in non-small-cell lung cancer: prognostic impact and clinico-pathological correlations. Respir Res. 2016; 17:105.
Article21. Lee JS, Lim SM, Rha SY, et al. Prognostic implications of anaplastic lymphoma kinase gene aberrations in rhabdomyosarcoma; an immunohistochemical and fluorescence in situ hybridisation study. J Clin Pathol. 2014; 67:33–9.
Article22. Schoppmann SF, Streubel B, Birner P. Amplification but not translocation of anaplastic lymphoma kinase is a frequent event in oesophageal cancer. Eur J Cancer. 2013; 49:1876–81.
Article23. Del Grosso F, De Mariano M, Passoni L, Luksch R, Tonini GP, Longo L. Inhibition of N-linked glycosylation impairs ALK phosphorylation and disrupts pro-survival signaling in neuroblastoma cell lines. BMC Cancer. 2011; 11:525.
Article24. Masui K, Komori T, Kato Y, et al. Elevated TERT expression in TERT-wildtype adult diffuse gliomas: histological evaluation with a novel TERT-specific antibody. Biomed Res Int. 2018; 2018:7945845.
Article25. Leclerc C, Haeich J, Aulestia FJ, et al. Calcium signaling orchestrates glioblastoma development: facts and conjunctures. Biochim Biophys Acta. 2016; 1863:1447–59.
Article26. Nishi H, Nakada T, Kyo S, Inoue M, Shay JW, Isaka K. Hypoxia-inducible factor 1 mediates upregulation of telomerase (hTERT). Mol Cell Biol. 2004; 24:6076–83.
Article27. Alkhaibary A, Alassiri AH, AlSufiani F, Alharbi MA. Ki-67 labeling index in glioblastoma; does it really matter? Hematol Oncol Stem Cell Ther. 2019; 12:82–8.
Article28. Tsidulko AY, Kazanskaya GM, Kostromskaya DV, et al. Prognostic relevance of NG2/CSPG4, CD44 and Ki-67 in patients with glioblastoma. Tumour Biol. 2017; 39:1010428317724282.
Article29. Abdelzaher E. Glioblastoma multiforme, NOS [Internet]. Bingham Farms: PathologyOutlines.com;2020 [cited 2020 May 27]. Available from: https://www.pathologyoutlines.com/topic/cnstumorglioblastomagiantcell.html.30. Marzec M, Liu X, Wong W, et al. Oncogenic kinase NPM/ALK induces expression of HIF1alpha mRNA. Oncogene. 2011; 30:1372–8.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Alteration of Alk in Neuroblastoma
- Immunohistochemical expression of anaplastic lymphoma kinase in neuroblastoma and its relations with some clinical and histopathological features
- Analysis of the Cancer Genome Atlas Data to Determine the Prognostic Value of GABPB1L and TERT in Glioblastoma
- ALK Protein Expression Is Related to Neuroblastoma Aggressiveness But Is Not Independent Prognostic Factor
- Clinicopathological Analysis of Systemic Anaplastic Large Cell Lymphoma