J Korean Neurosurg Soc.
2018 Mar;61(2):186-193. 10.3340/jkns.2017.0404.019.
Cold Allodynia after C2 Root Resection in Sprague-Dawley Rats
- Affiliations
-
- 1Department of Neurosurgery, Cham Teun Teun Hospital, Daegu, Korea.
- 2Department of Neurosurgery, Kyungpook National University Hospital, Daegu, Korea. dccho@knu.ac.kr
- 3Department of Anesthesiology and Pain Medicine, School of Dentistry, Kyungpook National University, Daegu, Korea.
- KMID: 2408019
- DOI: http://doi.org/10.3340/jkns.2017.0404.019
Abstract
OBJECTIVE
The purpose of this study was to evaluate pain-related behaviors after bilateral C2 root resection and change in pain patterns in the suboccipital region in rats.
METHODS
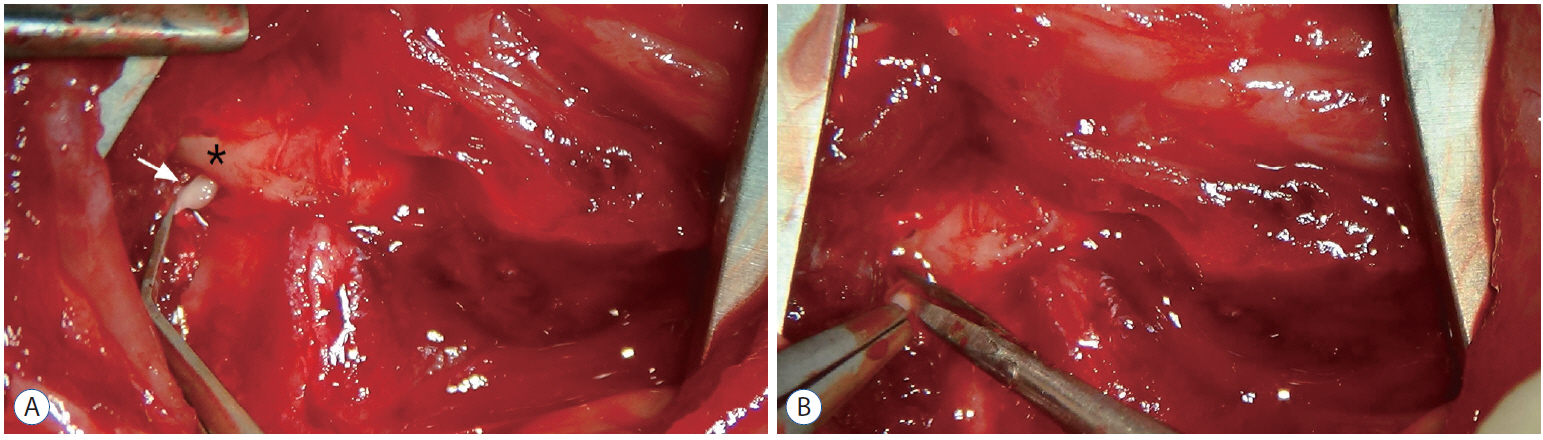
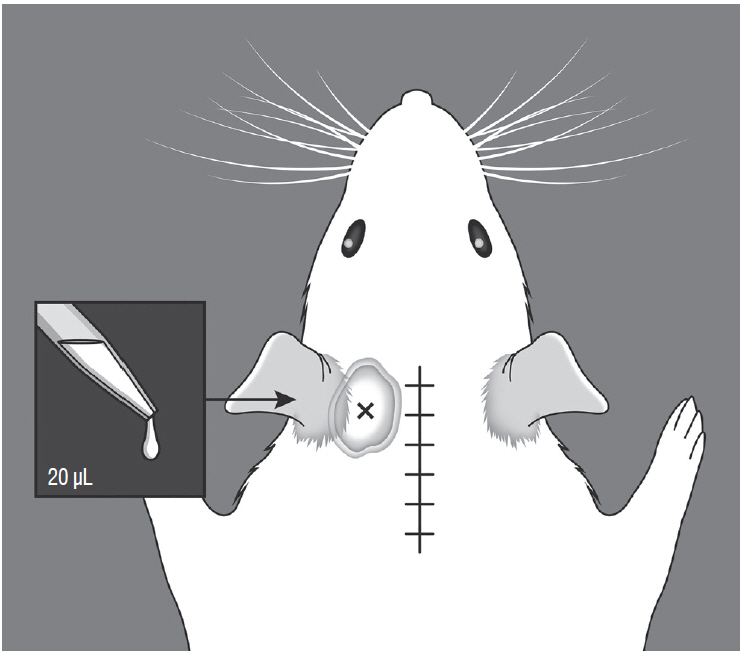
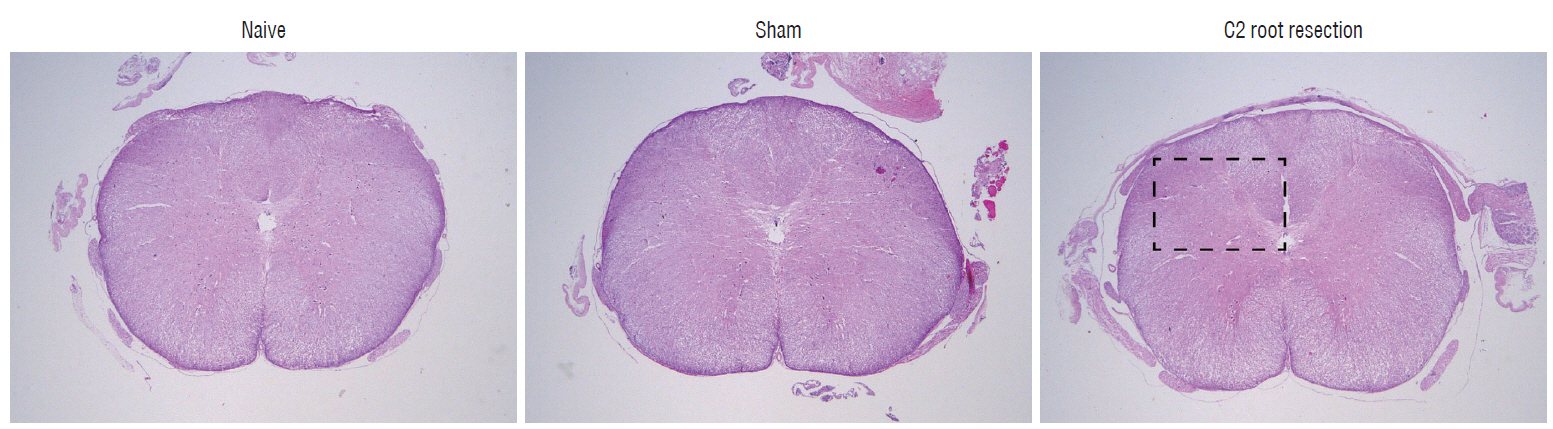
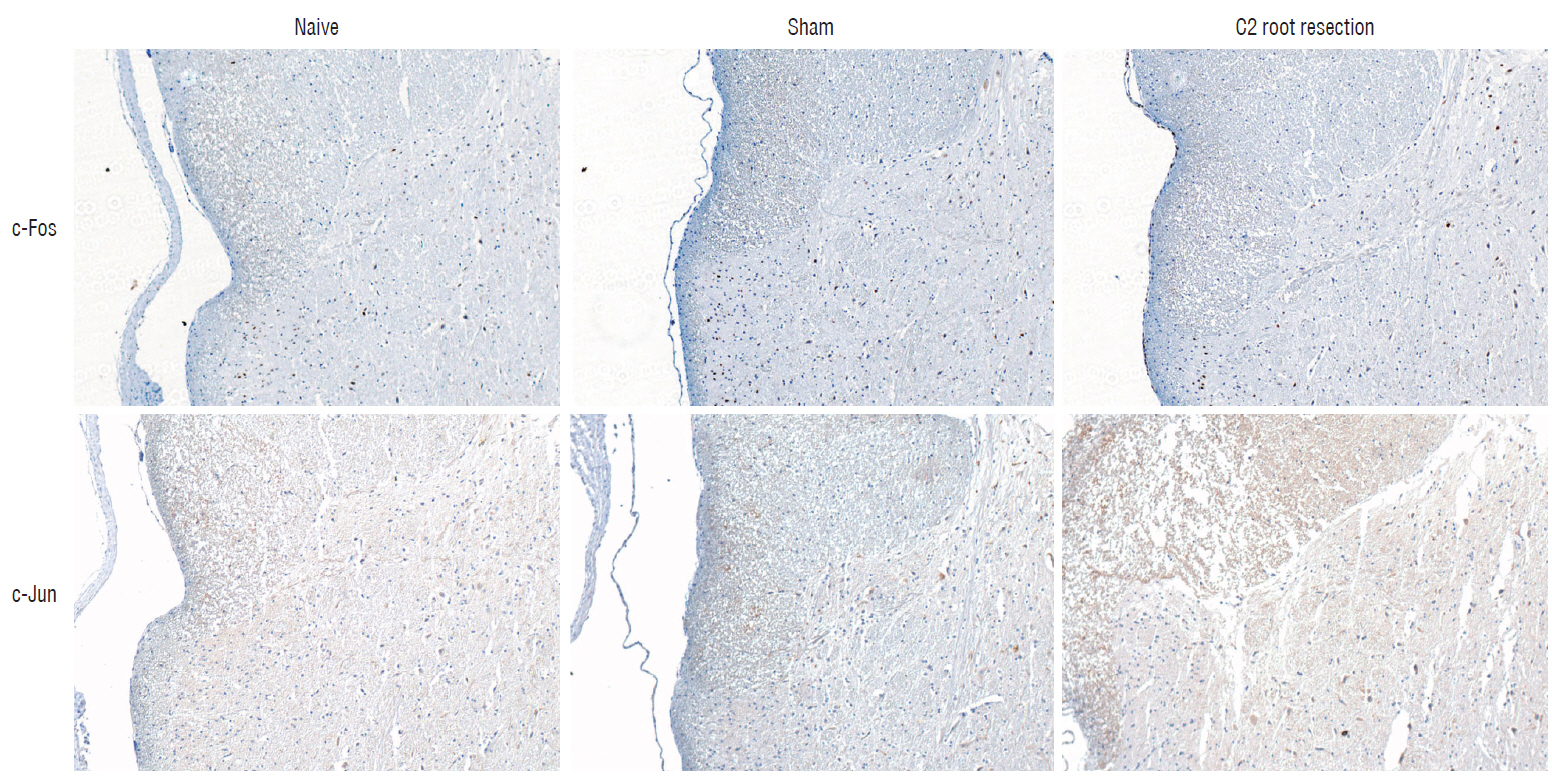
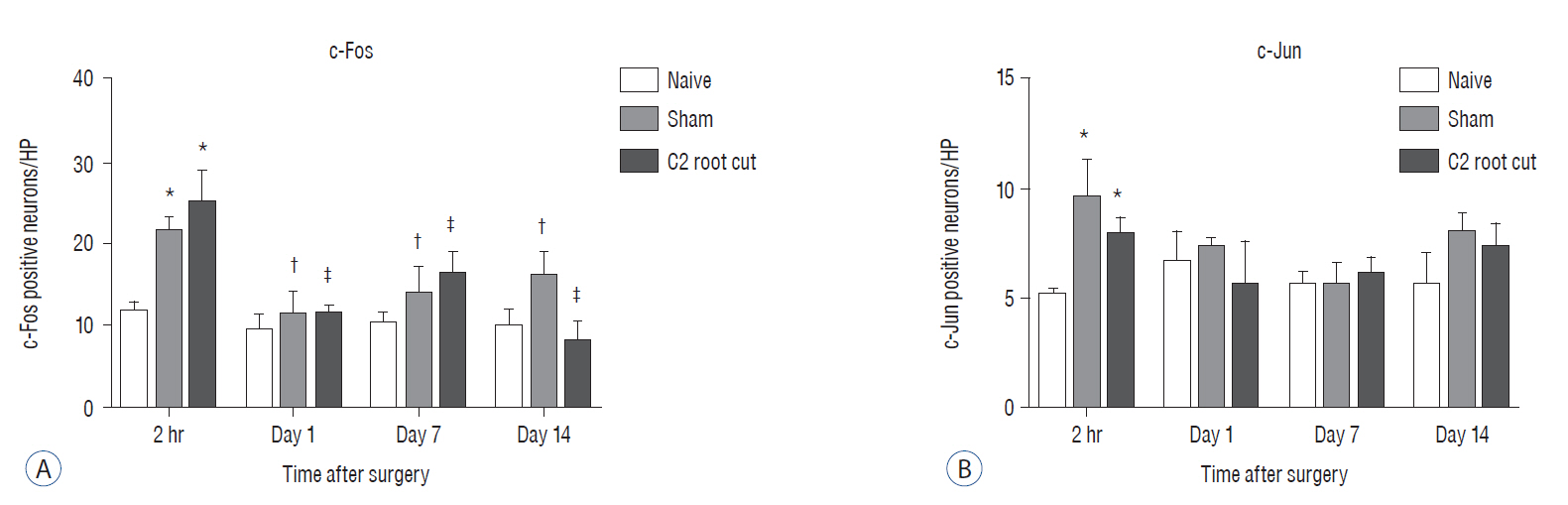
Male Sprague-Dawley rats were randomly assigned to three groups (n=25/group); näive, sham, and C2 resection. Three, 7, 10, and 14 days after surgery, cold allodynia was assessed using 20 μL of 99.7% acetone. c-Fos and c-Jun were immunohistochemically stained to evaluate activation of dorsal horn gray matter in C2 segments of the spinal cord 2 hours, 1 day, 7 days, and 14 days after surgery.
RESULTS
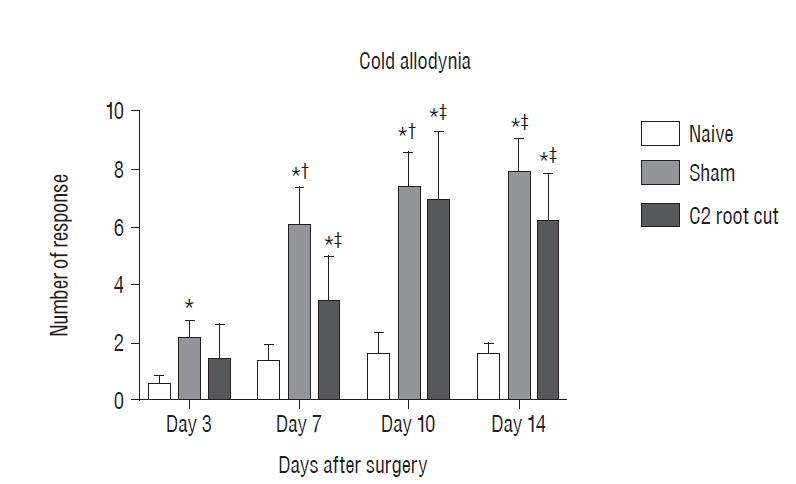
Three days after surgery, the response to acetone in the sham group was significantly greater than in the näive group, and this significant difference between the näive and sham groups was maintained throughout the experimental period (p < 0.05 at 3, 7, 10, and 14 days). Seven, 10, and 14 days after surgery, the C2 root resection group exhibited a significantly greater response to acetone than the näive group (p < 0.05), and both the sham and C2 resection groups exhibited significantly greater responses to acetone compared with 3 days after surgery. No significant difference in cold allodynia was observed between the sham and C2 root resection groups throughout the experimental period. Two hours after surgery, both the sham and C2 root resection groups exhibited significant increases in c-Fos- and c-Jun-positive neurons compared with the naive group (p=0.0021 and p=0.0358 for the sham group, and p=0.0135 and p=0.014 for the C2 root resection group, respectively). One day after surgery, both the sham and C2 root resection groups exhibited significant decreases in c-Fos -positive neurons compared with two hours after surgery (p=0.0169 and p=0.0123, respectively), and these significant decreases in c-Fos immunoreactivity were maintained in both the sham and C2 root resection groups 7 and 14 days after surgery. The sham and C2 root resection groups presented a tendency toward a decrease in c-Jun-positive neurons 1, 7, and 14 days after surgery, but the decrease did not reach statistical significance.
CONCLUSION
We found no significant difference in cold allodynia and the early expression of c-Fos and c-Jun between the sham and C2 resection groups. Our results may support the routine resection of the C2 nerve root for posterior C1-2 fusion, but, further studies are needed.
MeSH Terms
Figure
Reference
-
References
1. Ahn DK, Lim EJ, Kim BC, Yang GY, Lee MK, Ju JS, et al. Compression of the trigeminal ganglion produces prolonged nociceptive behavior in rats. Eur J Pain. 13:568–575. 2009.
Article2. Berrocal YA, Pearse DD, Andrade CM, Hechtman JF, Puentes R, Eaton MJ. Increased spinal c-Fos expression with noxious and non-noxious peripheral stimulation after severe spinal contusion. Neurosci Lett. 413:58–62. 2007.
Article3. Broude E, McAtee M, Kelley MS, Bregman BS. c-Jun expression in adult rat dorsal root ganglion neurons, differential response after central or peripheral axotomy. Exp Neurol. 148:367–377. 1997.
Article4. Campbell JN, Meyer RA. Mechanism of neuropathic pain. Neuron. 52:77–92. 2006.5. Catheline G, Le Guen S, Honoré P, Besson JM. Are there long-term changes in the basal or evoked Fos expression in the dorsal horn of the spinal cord of the mononeuropathic rat? Pain. 80:347–357. 1999.
Article6. Cohen SP, Mao J. Neuropathic pain: mechanisms and their clinical implications. BMJ. 348:f7656. 2014.
Article7. Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 87:149–158. 2000.
Article8. Ducati A. Nerves are not made to be cut. World Neurosurg. 78:601–602. 2012.
Article9. Elliott RE, Kang MM, Smith ML, Frempong-Boadu A. C2 nerve root sectioning in posterior atlantoaxial instrumented fusions: a structured review of literature. World Neurosurg. 78:697–708. 2012.
Article10. Elliott RE, Tanweer O, Boah A, Morsi A, Ma T, Smith ML, et al. Atlantoaxial fusion with screw-rod constructs: meta-analysis and review of literature. World Neurosurg. 81:411–421. 2014.
Article11. Goel A. Treatment of basilar invagination by atlantoaxial joint distraction and direct lateral mass fixation. J Neurosurg Spine. 1:281–286. 2004.
Article12. Goel A. Cervical ganglion 2 (CG2) neurectomy: a window to the atlantoaxial joint. World Neurosurg. 78:78–79. 2012.
Article13. Goel A, Desai KI, Muzumdar DP. Atlantoaxial fixation using plate and screw method: a report of 160 treated patients. Neurosurgery. 51:1351–1356. discussion 1356–1357. 2002.
Article14. Goel A, Laheri V. Plate and screw fixation for atlantoaxial subluxation. Acta Neurochir (Wien). 129:47–53. 1994.
Article15. Hamilton DK, Smith JS, Sansur CA, Dumont AS, Shaffrey CI. C-2 neurectomy during atlantoaxial instrumented fusion in the elderly: patient satisfaction and surgical outcome. J Neurosurg Spine. 15:3–8. 2011.
Article16. Harms J, Melcher RP. Posterior C1–C2 fusion with polyaxial screw and rod fixation. Spine (Phila Pa 1976). 26:2467–2471. 2001.
Article17. Harris JA. Using c-Fos as a neural marker of pain. Brain Res Bull. 45:1–8. 1998.
Article18. Jaggi AS, Jain V, Singh N. Animal models of neuropathic pain. Fundam Clin Pharmacol. 25:1–28. 2011.
Article19. Jeon HJ, Han SR, Park MK, Yang KY, Bae YC, Ahn DK. A novel trigeminal neuropathic pain model: compression of the trigeminal nerve root produces prolonged nociception in rats. Prog Neuropsychopharmacol Biol Psychiatry. 38:149–158. 2012.
Article20. Jeon Y, Kim CE, Jung D, Kwak K, Park S, Lim D, et al. Curcumin could prevent the development of chronic neuropathic pain in rats with peripheral nerve injury. Curr Ther Res Clin Exp. 74:1–4. 2013.
Article21. Kang MM, Anderer EG, Elliott RE, Kalhorn SP, Frempong-Boadu A. C2 nerve root sectioning in posterior C1–2 instrumented fusions. World Neurosurg. 78:170–177. 2012.
Article22. Kim IS, Hong JT, Sung JH, Byun JH. Verical reduction using atlantoaxial facet spacer in basilar invagination with atlantoaxial instability. J Korean Neurosurg Soc. 50:528–531. 2011.
Article23. Kim KJ, Yoon YW, Chung JM. Comparison of three rodent neuropathic pain models. Exp Brain Res. 113:200–206. 1997.
Article24. Klusáková I, Dubový P. Experimental models of peripheral neuropathic pain based on traumatic nerve injuries - an anatomical perspective. Ann Anat. 191:248–259. 2009.
Article25. Li H, Li Q, Xie K, Feng S, Wang P, Ma X. Expression of c-Fos and c-Jun in adjacent cervical spinal cord segments following C7 nerve root rhizotomy in rats: indication of a neural pathway between adjacent cervical spinal cord segments. Exp Ther Med. 6:373–377. 2013.
Article26. Loukas M, El-Sedfy A, Tubbs RS, Louis RG Jr, Wartmann CT, Curry B, et al. Identification of greater occipital nerve landmarks for the treatment of occipital neuralgia. Folia Morphol (Warsz). 65:337–342. 2006.27. McCoy ES, Zylka MJ. Enhanced behavioral responses to cold stimuli following CGRPα sensory neuron ablation are dependent on TRPM8. Mol Pain. 10:69. 2014.
Article28. Sideris A, Norcini M, Blanck TJ, Reico-Pinto E. Temporal and qualitative differences in the development of allodynic behaviors between mice and rats in a peripheral nerve injury model. Pain Studies and Treatment. 2:121. 2014.
Article29. Vanelderen P, Lataster A, Levy R, Mekhail N, van Kleef M, Van Zundert J. Occipital neuralgia. Pain Pract. 10:137–144. 2010.
Article30. Vital JM, Grenier F, Dautheribes M, Baspeyre H, Lavignolle B, Sénégas J. An anatomic and dynamic study of the greater occipital nerve (n. of Arnold) Applications to the treatment of Arnold’s neuralgia. Surg Radiol Anat. 11:205–210. 1989.
Article31. Wang TT, Yuan WL, Ke Q, Song XB, Zhou X, Kang Y, et al. Effects of electro-acupuncture on the expression of c-jun and c-fos in spared dorsal root ganglion and associated spinal laminae following removal of adjacent dorsal root ganglia in cats. Neuroscience. 140:1169–1176. 2006.
Article32. Yeom JS, Buchowski JM, Kim HJ, Chang BS, Lee CK, Riew KD. Postoperative occipital neuralgia with and without C2 nerve root transection during atlantoaxial screw fixation: a post-hoc comparative outcome study of prospectively collected data. Spine J. 13:786–795. 2013.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Causalgiform pain produced by the tight ligation of L5, L6 spinal nerves in the rat
- Does the Tibial and Sural Nerve Transection Model Represent Sympathetically Independent Pain?
- Tumor Necrosis Factor-alpha and Apoptosis Following Spinal Nerve Ligation Injury in Rats
- The Intracisternal Administration of MEK Inhibitor Attenuates Mechanical and Cold Allodynia in a Rat Model of Compression of the Trigeminal Ganglion
- Effects of Cervical Sympathectomy on Mechanical Allodynia and Cold Allodynia in a Rat Model of Neuropathic Pain