Yonsei Med J.
2006 Dec;47(6):847-851. 10.3349/ymj.2006.47.6.847.
Does the Tibial and Sural Nerve Transection Model Represent Sympathetically Independent Pain?
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine and Anesthesia and Pain Research Institute, Yonsei University College of Medicine, Seoul, Korea. ywleepain@yumc.yonsei.ac.kr
- KMID: 1777175
- DOI: http://doi.org/10.3349/ymj.2006.47.6.847
Abstract
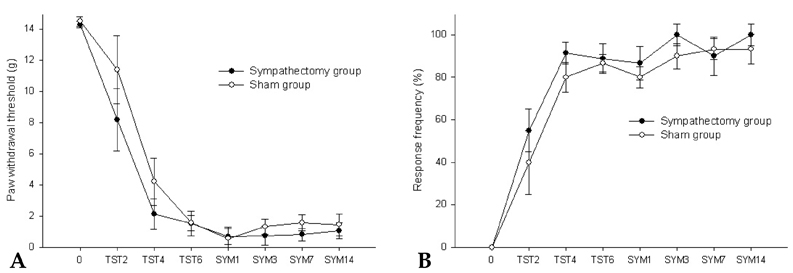
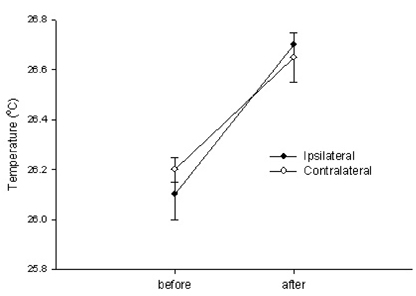
- Neuropathic pain can be divided into sympathetically maintained pain (SMP) and sympathetically independent pain (SIP). Rats with tibial and sural nerve transection (TST) produce neuropathic pain behaviors, including spontaneous pain, tactile allodynia, and cold allodynia. The present study was undertaken to examine whether rats with TST would represent SMP- or SIP-dominant neuropathic pain by lumbar surgical sympathectomy. The TST model was generated by transecting the tibial and sural nerves, leaving the common peroneal nerve intact. Animals were divided into the sympathectomy group and the sham group. For the sympathectomy group, the sympathetic chain was removed bilaterally from L2 to L6 one week after nerve transection. The success of the sympathectomy was verified by measuring skin temperature on the hind paw and by infra red thermography. Tactile allodynia was assessed using von Frey filaments, and cold allodynia was assessed using acetone drops. A majority of the rats exhibited withdrawal behaviors in response to tactile and cold stimulations after nerve stimulation. Neither tactile allodynia nor cold allodynia improved after successful sympathectomy, and there were no differences in the threshold of tactile and cold allodynia between the sympathectomy and sham groups. Tactile allodynia and cold allodynia in the neuropathic pain model of TST are not dependent on the sympathetic nervous system, and this model can be used to investigate SIP syndromes.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Antiallodynic Effect of Pregabalin in Rat Models of Sympathetically Maintained and Sympathetic Independent Neuropathic Pain
Dong Woo Han, Tae Dong Kweon, Jong Seok Lee, Youn-Woo Lee
Yonsei Med J. 2007;48(1):41-47. doi: 10.3349/ymj.2007.48.1.41.
Reference
-
1. Wahren LK, Torebjork E, Nystrom B. Quantitative sensory testing before and after regional guanethidine block in patients with neuralgia in the hand. Pain. 1991. 46:23–30.2. Kim SH, Na HS, Sheen K, Chung JM. Effects of sympathectomy on a rat model of peripheral neuropathy. Pain. 1993. 55:85–92.3. Lee BH, Won R, Baik EJ, Lee SH, Moon CH. An animal model of neuropathic pain employing injury to the sciatic nerve branches. Neuroreport. 2000. 11:657–661.4. Baron R, Janig W, Kollmann W. Sympathetic and afferent somata projecting in hindlimb nerves and the anatomical organization of the lumbar sympathetic nervous system of the rat. J Comp Neurol. 1988. 275:460–468.5. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994. 53:55–63.6. Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994. 59:369–376.7. Chung K, Lee BH, Yoon YW, Chung JM. Sympathetic sprouting in the dorsal root ganglia of the injured peripheral nerve in a rat neuropathic pain model. J Comp Neurol. 1996. 376:241–252.8. Kim KJ, Yoon YW, Chung JM. Comparison of three rodent neuropathic pain models. Exp Brain Res. 1997. 113:200–206.9. Lee BH, Yoon YW, Chung K, Chung JM. Comparison of sympathetic sprouting in sensory ganglia in three animal models of neuropathic pain. Exp Brain Res. 1998. 120:432–438.10. Dowdall T, Robinson I, Meert TF. Comparison of five different rat models of peripheral nerve injury. Pharmacol Biochem Behav. 2005. 80:93–108.11. McLachlan EM, Janig W, Devor M, Michaelis M. Peripheral nerve injury triggers noradrenergic sprouting within dorsal root ganglia. Nature. 1993. 363:543–546.12. Xie J, Park SK, Chung K, Chung JM. The effect of lumbar sympathectomy in the spinal nerve ligation model of neuropathic pain. J Pain. 2001. 2:270–278.13. Willenbring S, DeLeo JA, Coombs DW. Differential behavioral outcomes in the sciatic cryoneurolysis model of neuropathic pain in rats. Pain. 1994. 58:135–140.14. Shir Y, Seltzer Z. Effects of sympathectomy in a model of causalgiform pain produced by partial sciatic nerve injury in rats. Pain. 1991. 45:309–320.15. Neil A, Attal N, Guilbaud G. Effects of guanethidine on sensitization to natural stimuli and self-mutilating behaviour in rats with a peripheral neuropathy. Brain Res. 1991. 565:237–246.16. Korenman EM, Devor M. Ectopic adrenergic sensitivity in damaged peripheral nerve axons in the rat. Exp Neurol. 1981. 72:63–81.17. Lee DH, Katner J, Iyengar S, Lodge D. The effect of lumbar sympathectomy on increased tactile sensitivity in spinal nerve ligated rats. Neurosci Lett. 2001. 298:99–102.18. Cousins MJ, Reeve TS, Glynn CJ, Walsh JA, Cherry DA. Neurolytic lumbar sympathetic blockade: duration of denervation and relief of rest pain. Anaesth Intensive Care. 1979. 7:121–135.19. McCollum PT, Spence VA, Macrae B, Walker WF. Quantitative assessment of the effectiveness of chemical lumbar sympathectomy. Br J Anaesth. 1985. 57:1146–1149.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of MK-801 and Naloxone in a Sympathetically Independent Neuropathic Pain Rat Model
- Effect of Partial Sciatic Nerve Injury on the Response to Formalin Test in Rats
- The Expression of the Ca++ Channel alpha2delta Subunit and TRPM8 in the Dorsal Root Ganglion of Sympathetically Maintained Pain and Sympathetic Independent Pain Rat Models
- Antiallodynic Effect of Pregabalin in Rat Models of Sympathetically Maintained and Sympathetic Independent Neuropathic Pain
- The relationship between nerve conduction studies and neuropathic pain in sciatic nerve injury due to intramuscular injection