J Vet Sci.
2018 Mar;19(2):242-250. 10.4142/jvs.2018.19.2.242.
Rapid visual detection of Mycobacterium avium subsp. paratuberculosis by recombinase polymerase amplification combined with a lateral flow dipstick
- Affiliations
-
- 1Key Laboratory of Animal Resistant Biology of Shandong, College of Life Science, Shandong Normal University, Jinan 250014, China. hongbinhe@sdnu.edu.cn
- 2Ruminant Disease Research Center, Shandong Normal University, Jinan 250014, China.
- KMID: 2407623
- DOI: http://doi.org/10.4142/jvs.2018.19.2.242
Abstract
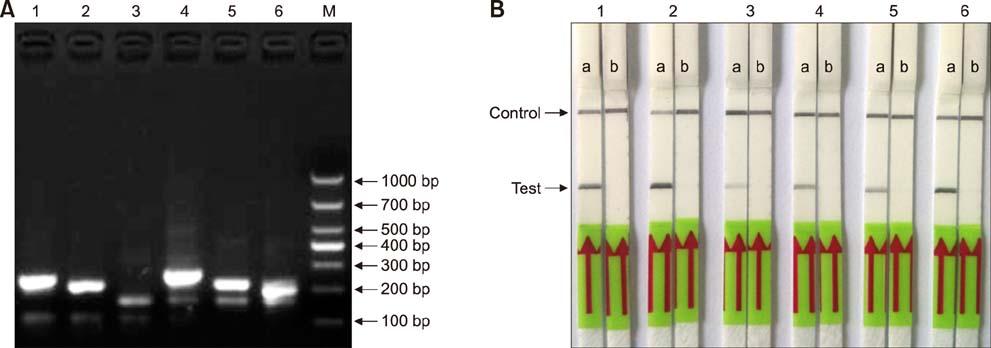
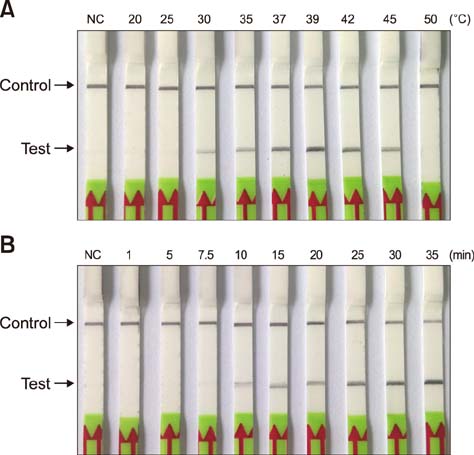
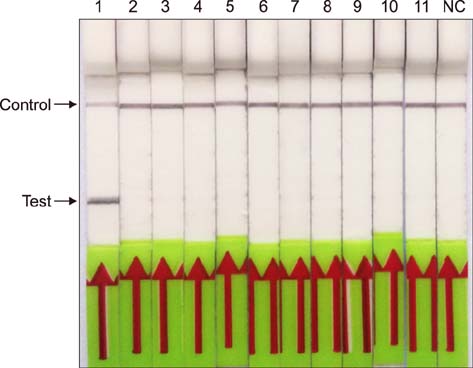
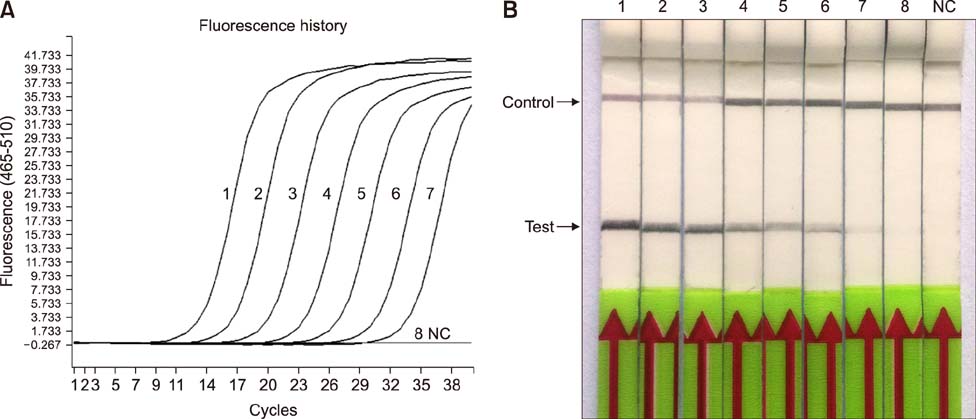
- Paratuberculosis (Johne's disease) is a chronic debilitating disease of domestic and wild ruminants. However, widespread point-of-care testing is infrequent due to the lack of a robust method. The isothermal recombinase polymerase amplification (RPA) technique has applied for rapid diagnosis. Herein, RPA combined with a lateral flow dipstick (LFD) assay was developed to estimate DNA from Mycobacterium avium subsp. paratuberculosis. First, analytical specificity and sensitivity of the RPA-nfo primer and probe sets were assessed. The assay successfully detected M. paratuberculosis DNA in 30 min at 39℃ with a detection limit of up to eight copies per reaction, which was equivalent to that of the real-time quantitative polymerase chain reaction (qPCR) assay. The assay was specific, as it did not amplify genomes from five other Mycobacterium spp. or five pathogenic enteric bacteria. Six hundred-twelve clinical samples (320 fecal and 292 serum) were assessed by RPA-LFD, qPCR, and enzyme-linked immunosorbent assay, respectively. The RPA-LFD assay yielded 100% sensitivity, 97.63% specificity, and 98.44% concordance rate with the qPCR results. This is the first report utilizing an RPA-LFD assay to visualize and rapidly detect M. paratuberculosis. Our results show this assay should be a useful method for the diagnosis of paratuberculosis in resource-constrained settings.
Keyword
MeSH Terms
-
Animals
Diagnosis
DNA
Enterobacteriaceae
Enzyme-Linked Immunosorbent Assay
Genome
Limit of Detection
Methods
Mycobacterium avium subsp. paratuberculosis*
Mycobacterium avium*
Mycobacterium*
Paratuberculosis
Point-of-Care Testing
Polymerase Chain Reaction
Recombinases*
Ruminants
Sensitivity and Specificity
DNA
Recombinases
Figure
Reference
-
1. Abd El, Patel P, Faye O, Thaloengsok S, Heidenreich D, Matangkasombut P, Manopwisedjaroen K, Sakuntabhai A, Sall AA, Hufert FT, Weidmann M. Recombinase polymerase amplification assay for rapid diagnostics of dengue infection. PLoS One. 2015; 10:e0129682.
Article2. Chaubey KK, Gupta RD, Gupta S, Singh SV, Bhatia AK, Jayaraman S, Kumar N, Goel A, Rathore AS, Sahzad , Sohal JS, Stephen BJ, Singh M, Goyal M, Dhama K, Derakhshandeh A. Trends and advances in the diagnosis and control of paratuberculosis in domestic livestock. Vet Q. 2016; 36:203–227.
Article3. Crannell ZA, Castellanos-Gonzalez A, Irani A, Rohrman B, White AC, Richards-Kortum R. Nucleic acid test to diagnose cryptosporidiosis: lab assessment in animal and patient specimens. Anal Chem. 2014; 86:2565–2571.
Article4. Daher RK, Stewart G, Boissinot M, Bergeron MG. Recombinase polymerase amplification for diagnostic applications. Clin Chem. 2016; 62:947–958.
Article5. Garcia AB, Shalloo L. The economic impact and control of paratuberculosis in cattle. J Dairy Sci. 2015; 98:5019–5039.6. Geraghty T, Graham DA, Mullowney P, More SJ. A review of bovine Johne's disease control activities in 6 endemically infected countries. Prev Vet Med. 2014; 116:1–11.
Article7. Hansen S, Schäfer J, Fechner K, Czerny CP, Abd El. Development of a recombinase polymerase amplification assay for rapid detection of the Mycobacterium avium subsp. paratuberculosis. PLoS One. 2016; 11:e0168733.8. Hou P, Wang H, Zhao G, He C, He H. Rapid detection of infectious bovine rhinotracheitis virus using recombinase polymerase amplification assays. BMC Vet Res. 2017; 13:386.
Article9. Hou P, Zhao G, He C, Wang H, He H. Biopanning of polypeptides binding to bovine ephemeral fever virus G1 protein from phage display peptide library. BMC Vet Res. 2018; 14:3.
Article10. Husakova M, Dziedzinska R, Slana I. Magnetic separation methods for the detection of Mycobacterium avium subsp. paratuberculosis in various types of matrices: a review. Biomed Res Int. 2017; 2017:5869854.11. Kersting S, Rausch V, Bier FF, von Nickisch-Rosenegk M. Rapid detection of Plasmodium falciparum with isothermal recombinase polymerase amplification and lateral flow analysis. Malar J. 2014; 13:99.
Article12. Liu W, Liu HX, Zhang L, Hou XX, Wan KL, Hao Q. A novel isothermal assay of Borrelia burgdorferi by recombinase polymerase amplification with lateral flow detection. Int J Mol Sci. 2016; 17:E1250.13. Meng QF, Li Y, Yang F, Yao GZ, Qian AD, Wang WL, Cong W. Seroprevalence and risk factors of Mycobacterium avium subspecies paratuberculosis infection in domestic sika deer in China. Trop Anim Health Prod. 2015; 47:999–1003.
Article14. Piepenburg O, Williams CH, Stemple DL, Armes NA. DNA detection using recombination proteins. PLoS Biol. 2006; 4:e204.
Article15. Song L, Zhang H, Hou P, Wang H, Zhao G, Xia X, He H. Development and preliminary application of an indirect ELISA to detect infectious bovine rhinotracheitis virus using recombinant glycoprotein D of IBRV strain SD. Kafkas Univ Vet Fak Derg. 2016; 22:503–509.16. Sun K, Xing W, Yu X, Fu W, Wang Y, Zou M, Luo Z, Xu D. Recombinase polymerase amplification combined with a lateral flow dipstick for rapid and visual detection of Schistosoma japonicum. Parasit Vectors. 2016; 9:476.
Article17. Tu PA, Shiu JS, Lee SH, Pang VF, Wang DC, Wang PH. Development of a recombinase polymerase amplification lateral flow dipstick (RPA-LFD) for the field diagnosis of caprine arthritis-encephalitis virus (CAEV) infection. J Virol Methods. 2017; 243:98–104.
Article18. Yang M, Ke Y, Wang X, Ren H, Liu W, Lu H, Zhang W, Liu S, Chang G, Tian S, Wang L, Huang L, Liu C, Yang R, Chen Z. Development and evaluation of a rapid and sensitive EBOV-RPA test for rapid diagnosis of Ebola virus disease. Sci Rep. 2016; 6:26943.
Article19. Yang Y, Qin X, Wang G, Jin J, Shang Y, Zhang Z. Development of an isothermoal amplification-based assay for rapid visual detection of an Orf virus. Virol J. 2016; 13:46.
Article20. Yang Y, Qin X, Zhang W, Li Y, Zhang Z. Rapid and specific detection of porcine parvovirus by isothermal recombinase polymerase amplification assays. Mol Cell Probes. 2016; 30:300–305.
Article21. Yue R, Liu C, Barrow P, Liu F, Cui Y, Yang L, Zhao D, Zhou X. The isolation and molecular characterization of Mycobacterium avium subsp. paratuberculosis in Shandong province, China. Gut Pathog. 2016; 8:9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effective DNA extraction method to improve detection of Mycobacterium avium subsp. paratuberculosis in bovine feces
- A biosensor assay for the detection of Mycobacterium avium subsp. paratuberculosis in fecal samples
- Tactics of Mycobacterium avium subsp. paratuberculosis for intracellular survival in mononuclear phagocytes
- Development of vaccines to Mycobacterium avium subsp. paratuberculosis infection
- Rapid identification of mycobacterium avium and mycobacterium intracellulare by the amplification of rRNA sequences