J Breast Cancer.
2015 Sep;18(3):242-248. 10.4048/jbc.2015.18.3.242.
The Presence of EpCAM-/CD49f+ Cells in Breast Cancer Is Associated with a Poor Clinical Outcome
- Affiliations
-
- 1Laboratory of Pathology, West China Hospital, Sichuan University, Chengdu, China. molecularpathology@hotmail.com
- 2Department of Pathology, West China Hospital, Sichuan University, Chengdu, China.
- KMID: 2407571
- DOI: http://doi.org/10.4048/jbc.2015.18.3.242
Abstract
- PURPOSE
It is well established that breast cancer stem cells (BCSCs) play an essential role in tumor invasion for both local and distant metastasis. The aim of this study was to establish whether BCSCs could act as a prognostic and clinical marker.
METHODS
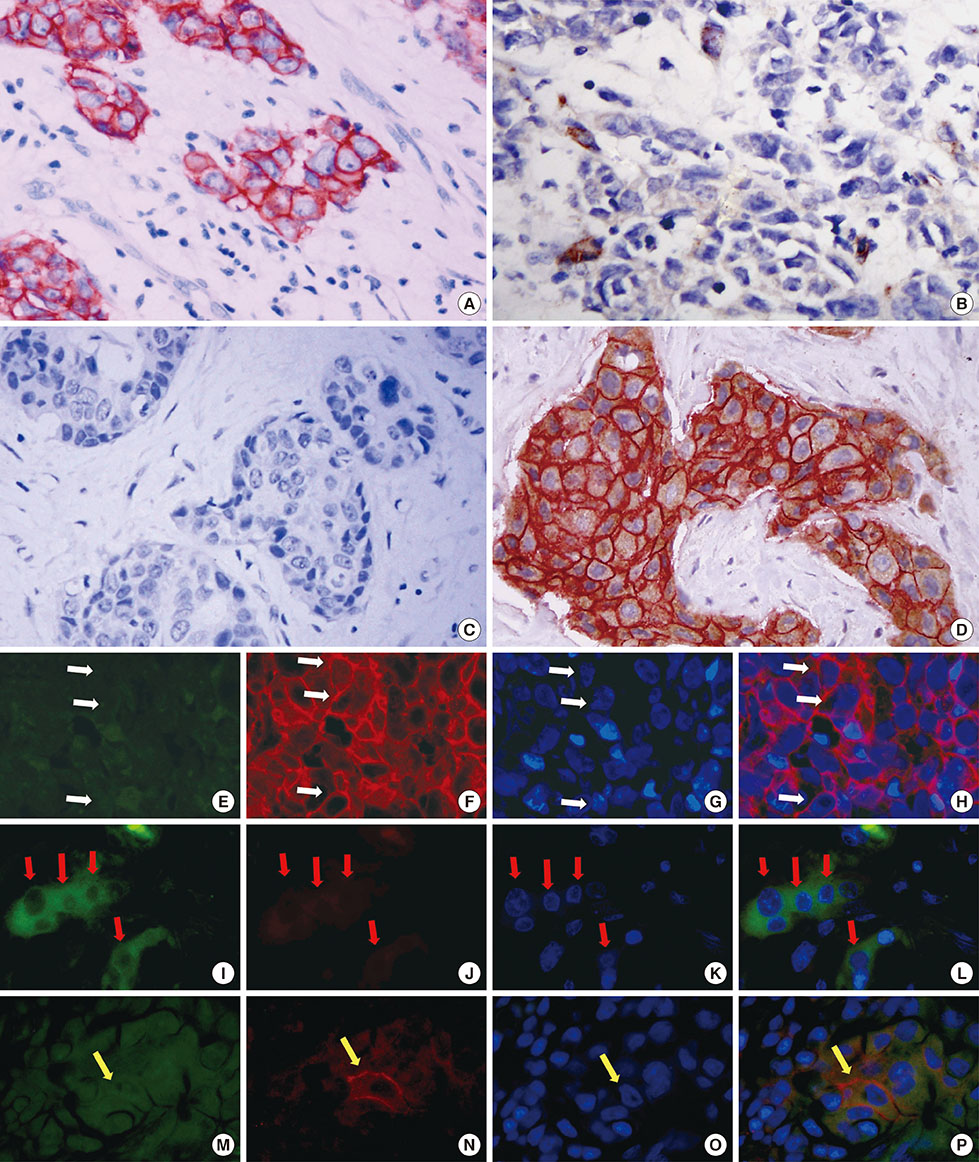
We analyzed tumor tissues from 161 breast cancer patients. Dual immunohistochemistry and immunofluorescence were performed on all the slides, and we analyzed the relationship between EpCAM-/CD49f+ tumor cells and key clinical and prognostic factors.
RESULTS
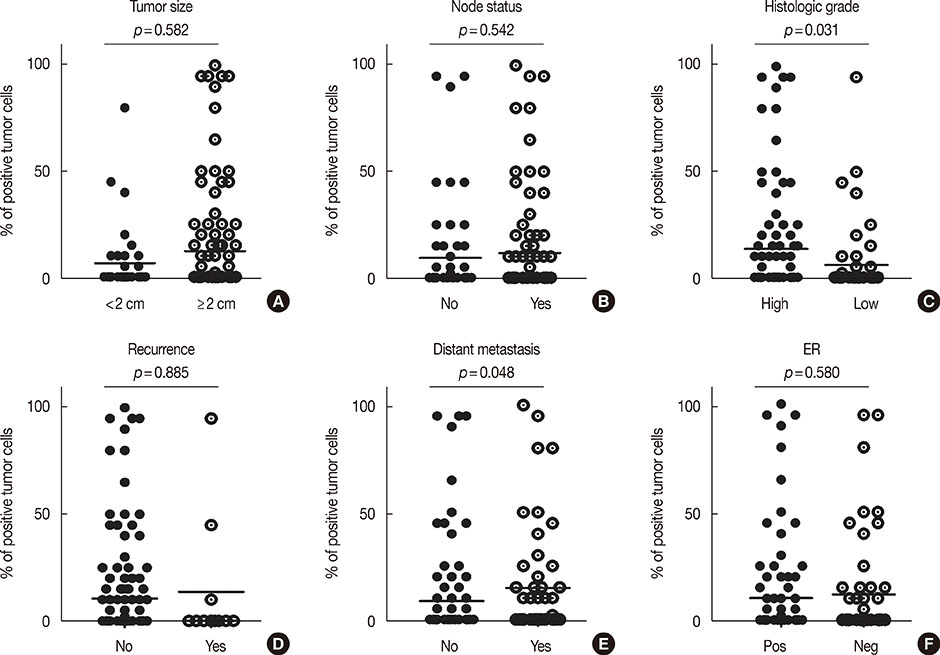
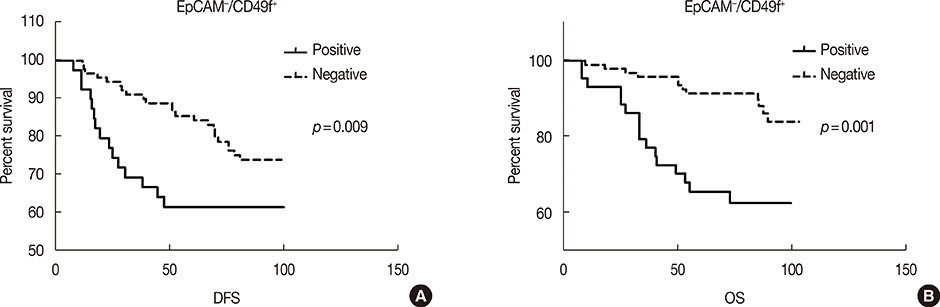
Univariate survival analysis using the Kaplan-Meier method showed that the presence of EpCAM-/CD49f+ tumor cells in breast cancer was significantly associated with shorter disease-free survival (DFS) and overall survival (OS). Multivariate analysis showed that the presence of EpCAM-/CD49f+ cells was associated with shorter DFS (p=0.010; hazard ratio [HR], 2.070) and OS (p=0.002; HR, 3.235). Tumors containing EpCAM-/CD49f+ cells were also more likely to metastasize after initial surgery (p=0.048).
CONCLUSION
Our study suggests that breast tumors containing EpCAM-/CD49f+ cells are more likely to undergo distant metastasis after initial surgery and are associated with a shorter DFS and OS.
MeSH Terms
Figure
Cited by 1 articles
-
CD49f Can Act as a Biomarker for Local or Distant Recurrence in Breast Cancer
Feng Ye, Xiaorong Zhong, Yan Qiu, Libo Yang, Bing Wei, Zhang Zhang, Hong Bu
J Breast Cancer. 2017;20(2):142-149. doi: 10.4048/jbc.2017.20.2.142.
Reference
-
1. Weigelt B, Geyer FC, Reis-Filho JS. Histological types of breast cancer: how special are they? Mol Oncol. 2010; 4:192–208.
Article2. Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003; 100:3983–3988.
Article3. Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007; 1:555–567.
Article4. Basak SK, Veena MS, Oh S, Huang G, Srivatsan E, Huang M, et al. The malignant pleural effusion as a model to investigate intratumoral heterogeneity in lung cancer. PLoS One. 2009; 4:e5884.
Article5. Huang EH, Hynes MJ, Zhang T, Ginestier C, Dontu G, Appelman H, et al. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009; 69:3382–3389.
Article6. Boonyaratanakornkit JB, Yue L, Strachan LR, Scalapino KJ, LeBoit PE, Lu Y, et al. Selection of tumorigenic melanoma cells using ALDH. J Invest Dermatol. 2010; 130:2799–2808.
Article7. Clay MR, Tabor M, Owen JH, Carey TE, Bradford CR, Wolf GT, et al. Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck. 2010; 32:1195–1201.
Article8. Deng S, Yang X, Lassus H, Liang S, Kaur S, Ye Q, et al. Distinct expression levels and patterns of stem cell marker, aldehyde dehydrogenase isoform 1 (ALDH1), in human epithelial cancers. PLoS One. 2010; 5:e10277.
Article9. Li T, Su Y, Mei Y, Leng Q, Leng B, Liu Z, et al. ALDH1A1 is a marker for malignant prostate stem cells and predictor of prostate cancer patients' outcome. Lab Invest. 2010; 90:234–244.
Article10. Marcato P, Dean CA, Giacomantonio CA, Lee PW. Aldehyde dehydrogenase: its role as a cancer stem cell marker comes down to the specific isoform. Cell Cycle. 2011; 10:1378–1384.
Article11. Sampieri K, Fodde R. Cancer stem cells and metastasis. Semin Cancer Biol. 2012; 22:187–193.
Article12. Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008; 133:704–715.
Article13. Abraham BK, Fritz P, McClellan M, Hauptvogel P, Athelogou M, Brauch H. Prevalence of CD44+/CD24-/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin Cancer Res. 2005; 11:1154–1159.14. Stingl J, Raouf A, Emerman JT, Eaves CJ. Epithelial progenitors in the normal human mammary gland. J Mammary Gland Biol Neoplasia. 2005; 10:49–59.
Article15. Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH, et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med. 2009; 15:907–913.
Article16. Honeth G, Bendahl PO, Ringnér M, Saal LH, Gruvberger-Saal SK, Lövgren K, et al. The CD44+/CD24- phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008; 10:R53.17. Stecklein SR, Jensen RA, Pal A. Genetic and epigenetic signatures of breast cancer subtypes. Front Biosci (Elite Ed). 2012; 4:934–949.18. Mizejewski GJ. Role of integrins in cancer: survey of expression patterns. Proc Soc Exp Biol Med. 1999; 222:124–138.
Article19. Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008; 8:604–617.
Article20. Mukhopadhyay R, Theriault RL, Price JE. Increased levels of alpha6 integrins are associated with the metastatic phenotype of human breast cancer cells. Clin Exp Metastasis. 1999; 17:325–332.21. Mercurio AM, Rabinovitz I. Towards a mechanistic understanding of tumor invasion: lessons from the alpha6beta 4 integrin. Semin Cancer Biol. 2001; 11:129–141.22. Chakraborty AK, Funasaka Y, Ichihashi M, Pawelek JM. Upregulation of alpha and beta integrin subunits in metastatic macrophage-melanoma fusion hybrids. Melanoma Res. 2009; 19:343–349.
Article23. Kwon J, Lee TS, Lee HW, Kang MC, Yoon HJ, Kim JH, et al. Integrin alpha 6: a novel therapeutic target in esophageal squamous cell carcinoma. Int J Oncol. 2013; 43:1523–1530.24. Hoogland AM, Verhoef EI, Roobol MJ, Schröder FH, Wildhagen MF, van der Kwast TH, et al. Validation of stem cell markers in clinical prostate cancer: alpha6-integrin is predictive for non-aggressive disease. Prostate. 2014; 74:488–496.25. Steglich A, Vehlow A, Eke I, Cordes N. Alpha integrin targeting for radiosensitization of three-dimensionally grown human head and neck squamous cell carcinoma cells. Cancer Lett. 2015; 357:542–548.26. Haraguchi N, Ishii H, Mimori K, Ohta K, Uemura M, Nishimura J, et al. CD49f-positive cell population efficiently enriches colon cancer-initiating cells. Int J Oncol. 2013; 43:425–430.27. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009; 139:871–890.28. Chaves-Pérez A, Mack B, Maetzel D, Kremling H, Eggert C, Harréus U, et al. EpCAM regulates cell cycle progression via control of cyclin D1 expression. Oncogene. 2013; 32:641–650.29. Liu P, Wang Z, Brown S, Kannappan V, Tawari PE, Jiang W, et al. Liposome encapsulated Disulfiram inhibits NFkappaB pathway and targets breast cancer stem cells in vitro and in vivo. Oncotarget. 2014; 5:7471–7485.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immune Responses to Plant-Derived Recombinant Colorectal Cancer Glycoprotein EpCAM-FcK Fusion Protein in Mice
- CD49f Can Act as a Biomarker for Local or Distant Recurrence in Breast Cancer
- Immunohistochemical Expression and Clinical Significance of Suggested Stem Cell Markers in Hepatocellular Carcinoma
- Breast Cancer During Pregnancy
- The Expression of Aldehyde Dehydrogenase Family in Breast Cancer