Anesth Pain Med.
2017 Oct;12(4):326-334. 10.17085/apm.2017.12.4.326.
Propacetamol as an alternative of ketorolac for postoperative pain management using patient-controlled analgesia
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Daegu Fatima Hospital, Daegu, Korea. anji43@naver.com
- KMID: 2405849
- DOI: http://doi.org/10.17085/apm.2017.12.4.326
Abstract
- BACKGROUND
The objective of this study was to examine effect of propacetamol in comparison with ketorolac in intravenous patient-controlled analgesia after gynecologic surgeries.
METHODS
Patients aged 18 to 70 years and undergoing laparoscopic gynecologic surgeries were selected. They were randomly allocated to either group K (180 mg of ketorolac with fentanyl and ramosetron) or group P (10 g of propacetamol with fentanyl and ramosetron). Their vital signs and visual analogue scale (VAS) were examined six times (0 min, 15 min, 30 min, 60 min, 12 h, and 24 h) and laboratory workup was done 48 hours after PCA application. Development of side effects was examined 15 minutes after the PCA application. Data from 111 patients were used for the final analysis.
RESULTS
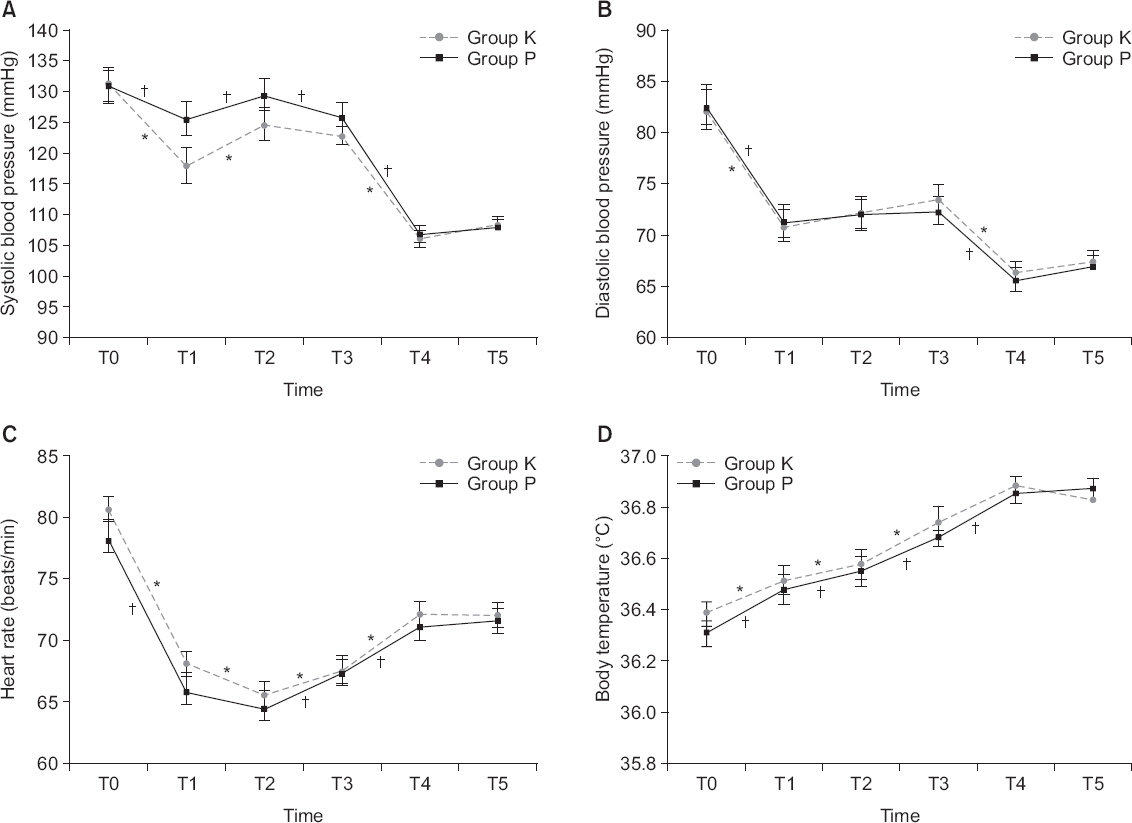
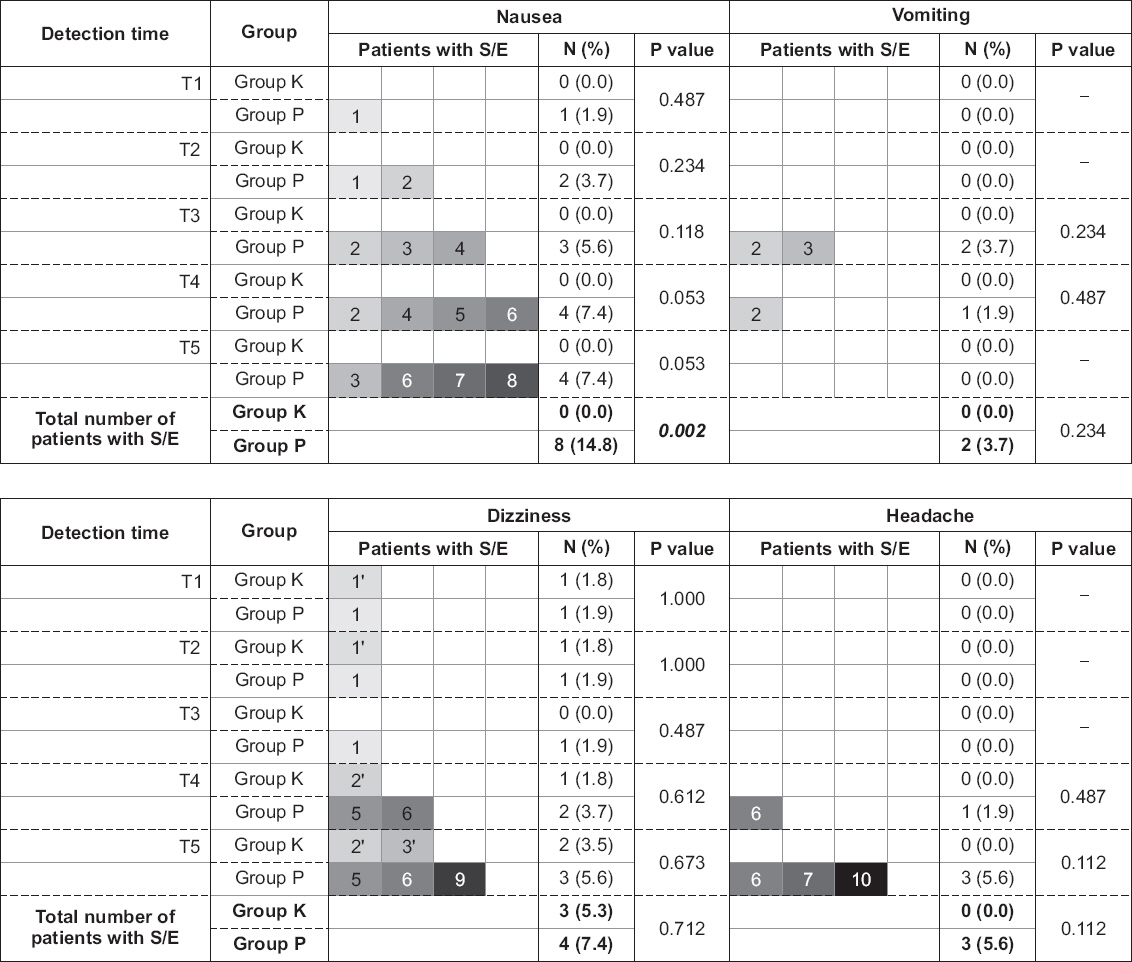
There were no significant differences in changes of systolic and diastolic blood pressures, heart rate, body temperature, and VAS between the groups (P = 0.325, 0.835, 0.346, 0.524, and 0.382, respectively). There were significant differences in the levels of hemoglobin, hematocrit, blood urea nitrogen, and international normalized ratio but it was not clinically meaningful. The development of vomiting, dizziness, and headache were not significantly different between the groups and no patient developed pruritus. Although the overall number of patients with nausea was higher in group P with statistical significance (P = 0.002), there were no significant differences between the groups when examined at each detection time.
CONCLUSIONS
The present study suggested propacetamol as a possible alternative of ketorolac in postoperative care after laparoscopic gynecologic surgeries.
Keyword
MeSH Terms
-
Analgesia
Analgesia, Patient-Controlled*
Blood Urea Nitrogen
Body Temperature
Dizziness
Female
Fentanyl
Gynecologic Surgical Procedures
Headache
Heart Rate
Hematocrit
Humans
International Normalized Ratio
Ketorolac*
Nausea
Pain, Postoperative*
Passive Cutaneous Anaphylaxis
Postoperative Care
Pruritus
Vital Signs
Vomiting
Fentanyl
Ketorolac
Figure
Reference
-
1. Hurley RW, Murphy JD, Wu CL. Miller RD, Erikson LI, Fleisher L, Wiener-Kronish PW, Cohen NH, Young WL, editors. Acute postoperative pain. Miller's Anesthesia. 8th ed. Philadelphia: Churchill Livingstone/Elsevier;2015. p. 2979.2. Butterworth JF, Mackey DC, Wasnick JD. Morgan and Mikhail's Clinical Anesthesia. 5th ed. New York: McGraw-Hill Education;2013. p. 190. PMID: 23657344.3. Marret E, Kurdi O, Zufferey P, Bonnet F. Effects of nonsteroidal antiinflammatory drugs on patient-controlled analgesia morphine side effects:meta-analysis of randomized controlled trials. Anesthesiology. 2005; 102:1249–60. DOI: 10.1097/00000542-200506000-00027. PMID: 15915040.4. Cepeda MS, Carr DB, Miranda N, Diaz A, Silva C, Morales O. Comparison of morphine, ketorolac, and their combination for postoperative pain:results from a large, randomized, double-blind trial. Anesthesiology. 2005; 103:1225–32. DOI: 10.1097/00000542-200512000-00018. PMID: 16306736.5. Myles PS, Power I. Clinical update:postoperative analgesia. Lancet. 2007; 369:810–2. DOI: 10.1016/S0140-6736(07)60388-2.6. Macario A, Lipman AG. Ketorolac in the era of cyclo-oxygenase-2 selective nonsteroidal anti-inflammatory drugs:a systematic review of efficacy, side effects, and regulatory issues. Pain Med. 2001; 2:336–51. DOI: 10.1046/j.1526-4637.2001.01043.x. PMID: 15102238.7. Marret E, Flahault A, Samama CM, Bonnet F. Effects of postoperative, nonsteroidal, antiinflammatory drugs on bleeding risk after tonsillectomy:meta-analysis of randomized, controlled trials. Anesthesiology. 2003; 98:1497–502. DOI: 10.1097/00000542-200306000-00030. PMID: 12766664.8. Gorissen KJ, Benning D, Berghmans T, Snoeijs MG, Sosef MN, Hulsewe KW, et al. Risk of anastomotic leakage with non-steroidal anti-inflammatory drugs in colorectal surgery. Br J Surg. 2012; 99:721–7. DOI: 10.1002/bjs.8691. PMID: 22318712.9. Busti AJ, Hooper JS, Amaya CJ, Kazi S. Effects of perioperative antiinflammatory and immunomodulating therapy on surgical wound healing. Pharmacotherapy. 2005; 25:1566–91. DOI: 10.1592/phco.2005.25.11.1566. PMID: 16232020.10. Lee A, Cooper MG, Craig JC, Knight JF, Keneally JP. The effects of nonsteroidal anti-inflammatory drugs (NSAIDs) on postoperative renal function:a meta-analysis. Anaesth Intensive Care. 1999; 27:574–80. PMID: 10631409.11. Catapano MS. The analgesic efficacy of ketorolac for acute pain. J Emerg Med. 1996; 14:67–75. DOI: 10.1016/0736-4679(95)02052-7.12. Delbos A, Boccard E. The morphine-sparing effect of propacetamol in orthopedic postoperative pain. J Pain Symptom Manage. 1995; 10:279–86. DOI: 10.1016/0885-3924(95)00004-I.13. Flouvat B, Leneveu A, Fitoussi S, Delhotal-Landes B, Gendron A. Bioequivalence study comparing a new paracetamol solution for injection and propacetamol after single intravenous infusion in healthy subjects. Int J Clin Pharmacol Ther. 2004; 42:50–7. DOI: 10.5414/CPP42050. PMID: 14756388.14. Campanero MA, Calahorra B, García-Quétglas E, López-Ocáriz A, Honorato J. Rapid liquid chromatographic assay for the determination of acetaminophen in plasma after propacetamol administration:application to pharmacokinetic studies. J Pharm Biomed Anal. 1999; 20:327–34. DOI: 10.1016/S0731-7085(99)00051-5.15. Bannwarth B, Netter P, Lapicque F, Gillet P, Péré P, Boccard E, et al. Plasma and cerebrospinal fluid concentrations of paracetamol after a single intravenous dose of propacetamol. Br J Clin Pharmacol. 1992; 34:79–81. DOI: 10.1111/j.1365-2125.1992.tb04112.x. PMID: 1633071. PMCID: PMC1381380.16. Smith HS. Potential analgesic mechanisms of acetaminophen. Pain Physician. 2009; 12:269–80. PMID: 19165309.17. Burian M, Geisslinger G. COX-dependent mechanisms involved in the antinociceptive action of NSAIDs at central and peripheral sites. Pharmacol Ther. 2005; 107:139–54. DOI: 10.1016/j.pharmthera.2005.02.004. PMID: 15993252.18. Woolf CJ, Chong MS. Preemptive analgesia--treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993; 77:362–79. DOI: 10.1213/00000539-199377020-00026.19. Desmeules J, Rollason V, Piguet V, Dayer P. Clinical pharmacology and rationale of analgesic combinations. Eur J Anaesthesiol Suppl. 2003; 28:7–11. PMID: 12785456.20. Heo BH, Park JH, Choi JI, Kim WM, Lee HG, Cho SY, et al. A comparative efficacy of propacetamol and ketorolac in postoperative patient controlled analgesia. Korean J Pain. 2015; 28:203–9. DOI: 10.3344/kjp.2015.28.3.203. PMID: 26175881. PMCID: PMC4500785.21. Pickering TG. Effects of stress and behavioral interventions in hypertension. Pain and blood pressure. J Clin Hypertens (Greenwich). 2003; 5:359–61. DOI: 10.1111/j.1524-6175.2003.02830.x.22. Terkelsen AJ, Mølgaard H, Hansen J, Andersen OK, Jensen TS. Acute pain increases heart rate:differential mechanisms during rest and mental stress. Auton Neurosci. 2005; 121:101–9. DOI: 10.1016/j.autneu.2005.07.001. PMID: 16081322.23. Held BI, Michels A, Blanco J, Ascher-Walsh C. The effect of ketorolac on postoperative febrile episodes in patients after abdominal myomectomy. Am J Obstet Gynecol. 2002; 187:1450–5. DOI: 10.1067/mob.2002.130006. PMID: 12501045.24. Kett DH, Breitmeyer JB, Ang R, Royal MA. A randomized study of the efficacy and safety of intravenous acetaminophen vs. intravenous placebo for the treatment of fever. Clin Pharmacol Ther. 2011; 90:32–9. DOI: 10.1038/clpt.2011.98. PMID: 21544074.25. Warwick C. Paracetamol and fever management. J R Soc Promot Health. 2008; 128:320–3. DOI: 10.1177/1466424008092794. PMID: 19058473.26. Varrassi G, Marinangeli F, Agrò F, Aloe L, De Cillis P, De Nicola A, et al. A double-blinded evaluation of propacetamol versus ketorolac in combination with patient-controlled analgesia morphine:analgesic efficacy and tolerability after gynecologic surgery. Anesth Analg. 1999; 88:611–6. DOI: 10.1097/00000539-199903000-00028. PMID: 10072016.27. Oöpik V, Saaremets I, Medijainen L, Karelson K, Janson T, Timpmann S. Effects of sodium citrate ingestion before exercise on endurance performance in well trained college runners. Br J Sports Med. 2003; 37:485–9. DOI: 10.1136/bjsm.37.6.485. PMID: 14665584. PMCID: PMC1724692.28. Kjaer K, Comerford M, Kondilis L, DiMaria L, Abramovitz S, Kiselev M, et al. Oral sodium citrate increases nausea amongst elective Cesarean delivery patients. Can J Anaesth. 2006; 53:776–80. DOI: 10.1007/BF03022794. PMID: 16873344.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Comparative Efficacy of Propacetamol and Ketorolac in Postoperative Patient Controlled Analgesia
- The effect of ketorolac and propacetamol on pain control after tonsillectomy in pediatric patients
- A Comparison of Butorphanol and Fentanyl Administered in Conjuction with Ketorolac in Intravenous Patient Controlled Analgesia after Total Abdominal Hysterectomy
- Intravenous Patient-controlled Analgesia Has a Positive Effect on the Prognosis of Delirium in Patients Undergoing Orthopedic Surgery
- A Effectiveness of Butorphanol and Nalbuphine as Utilized with Ketorolac in Patient Controlled Analgesia after Total Abdominal Hysterectomy