J Korean Neurosurg Soc.
2018 Jan;61(1):10-18. 10.3340/jkns.2017.0203.003.
Curcumin Increase the Expression of Neural Stem/Progenitor Cells and Improves Functional Recovery after Spinal Cord Injury
- Affiliations
-
- 1Department of Neurosurgery, Kyungpook National University Hospital, Daegu, Korea. nskimkt7@gmail.com
- 2Department of Neurosurgery, Seoul National University Hospital, Seoul, Korea.
- KMID: 2403518
- DOI: http://doi.org/10.3340/jkns.2017.0203.003
Abstract
OBJECTIVE
To investigates the effect of curcumin on proliferation of spinal cord neural stem/progenitor cells (SC-NSPCs) and functional outcome in a rat spinal cord injury (SCI) model.
METHODS
Sixty adult male Sprague-Dawley rats were randomly and blindly allocated into three groups (sham control group; curcumin treated group after SCI; vehicle treated group after SCI). Functional recovery was evaluated by the Basso, Beattie, and Bresnahan (BBB) scale during 6 weeks after SCI. The expression of SC-NSPC proliferation and astrogliosis were analyzed by nestin/Bromodeoxyuridine (BrdU) and Glial fibrillary acidic protein (GFAP) staining. The injured spinal cord was then examined histologically, including quantification of cavitation.
RESULTS
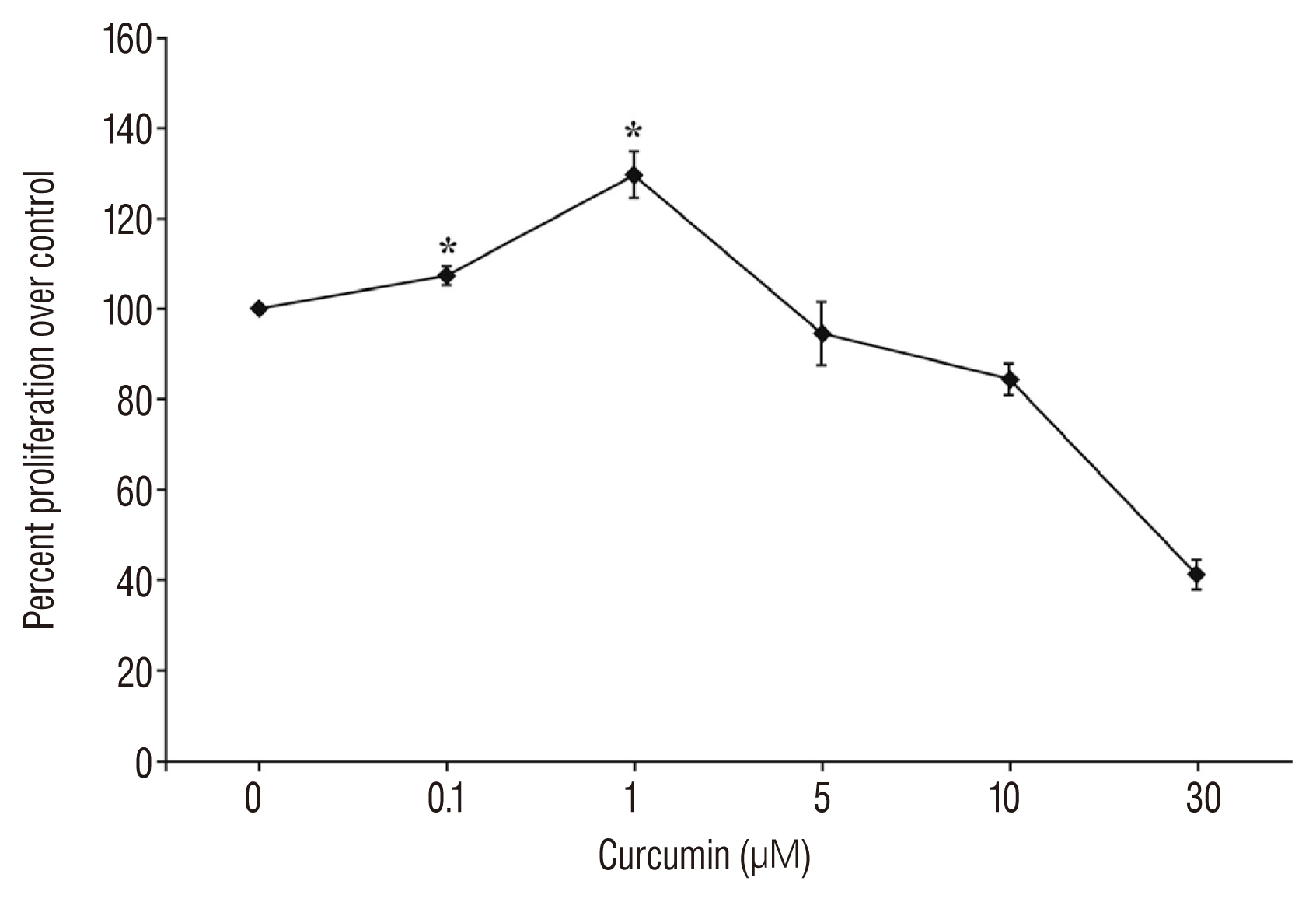
The BBB score of the SCI-curcumin group was better than that of SCI-vehicle group up to 14 days (p < 0.05). The co-immunoreactivity of nestin/BrdU in the SCI-curcumin group was much higher than that of the SCI-vehicle group 1 week after surgery (p < 0.05). The GFAP immunoreactivity of the SCI-curcumin group was remarkably lower than that of the SCI-vehicle group 4 weeks after surgery (p < 0.05). The lesion cavity was significantly reduced in the curcumin group as compared to the control group (p < 0.05).
CONCLUSION
These results indicate that curcumin could increase the expression of SC-NSPCs, and reduce the activity of reactive astrogliosis and lesion cavity. Consequently curcumin could improve the functional recovery after SCI via SC-NSPC properties.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Alvarez-Buylla A, García-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2:287–293. 2001.
Article2. Balentine JD. Pathology of experimental spinal cord trauma. I The necrotic lesion as a function of vascular injury. Lab Invest. 39:236–253. 1978.3. Balentine JD. Pathology of experimental spinal cord trauma. II Ultrastructure of axons and myelin. Lab Invest. 39:254–266. 1978.4. Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 139:244–256. 1996.
Article5. Bethea JR, Dietrich WD. Targeting the host inflammatory response in traumatic spinal cord injury. Curr Opin Neurol. 15:355–360. 2002.
Article6. Chen DY, Lu XH, Chen Y, Yan WJ, Yang HS, Wang XW, et al. Anterior decompression for the treatment of cervical spondylotic myelopathy associated with ossification of posterior longitudinal ligament. Zhonghua Wai Ke Za Zhi. 47:610–612. 2009.7. Chen F, Wang H, Xiang X, Yuan J, Chu W, Xue X, et al. Curcumin increased the differentiation rate of neurons in neural stem cells via wnt signaling in vitro study. J Surg Res. 192:298–304. 2014.
Article8. Chen Z, Liu B, Dong J, Feng F, Chen R, Xie P, et al. Comparison of anterior corpectomy and fusion versus laminoplasty for the treatment of cervical ossification of posterior longitudinal ligament: a meta-analysis. Neurosurg Focus. 40:E8. 2016.
Article9. Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 36:180–190. 2001.
Article10. Folwarczna J, Zych M, Trzeciak HI. Effects of curcumin on the skeletal system in rats. Pharmacol Rep. 62:900–909. 2010.
Article11. Gage FH, Coates PW, Palmer TD, Kuhn HG, Fisher LJ, Suhonen JO, et al. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci U S A. 92:11879–11883. 1995.
Article12. Houle JD, Tessler A. Repair of chronic spinal cord injury. Exp Neurol. 182:247–260. 2003.
Article13. Inoue T, Kawaguchi S, Kurisu K. Spontaneous regeneration of the pyramidal tract after transection in young rats. Neurosci Lett. 247:151–154. 1998.
Article14. Iwai H, Nori S, Nishimura S, Yasuda A, Takano M, Tsuji O, et al. Transplantation of neural stem/progenitor cells at different locations in mice with spinal cord injury. Cell Transplant. 23:1451–1464. 2014.
Article15. Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisén J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 96:25–34. 1999.
Article16. Kim KT, Kim HJ, Cho DC, Bae JS, Park SW. Substance P stimulates proliferation of spinal neural stem cells in spinal cord injury via the mitogen-activated protein kinase signaling pathway. Spine J. 15:2055–2065. 2015.
Article17. Kim SJ, Son TG, Park HR, Park M, Kim MS, Kim HS, et al. Curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult hippocampus. J Biol Chem. 283:14497–14505. 2008.
Article18. Kordek R, Nerurkar VR, Liberski PP, Isaacson S, Yanagihara R, Gajdusek DC. Heightened expression of tumor necrosis factor alpha, interleukin 1 alpha, and glial fibrillary acidic protein in experimental creutzfeldt-jakob disease in mice. Proc Natl Acad Sci U S A. 93:9754–9758. 1996.
Article19. Li J, Lepski G. Cell transplantation for spinal cord injury: a systematic review. Biomed Res Int. 2013:786475. 2013.
Article20. Lin HW, Basu A, Druckman C, Cicchese M, Krady JK, Levison SW. Astrogliosis is delayed in type 1 interleukin-1 receptor-null mice following a penetrating brain injury. J Neuroinflammation. 3:15. 2006.
Article21. Lin MS, Lee YH, Chiu WT, Hung KS. Curcumin provides neuroprotection after spinal cord injury. J Surg Res. 166:280–289. 2011.
Article22. McNally SJ, Harrison EM, Ross JA, Garden OJ, Wigmore SJ. Curcumin induces heme oxygenase 1 through generation of reactive oxygen species, p38 activation and phosphatase inhibition. Int J Mol Med. 19:165–172. 2007.
Article23. Milligan ED, Hinde JL, Mehmert KK, Maier SF, Watkins LR. A method for increasing the viability of the external portion of lumbar catheters placed in the spinal subarachnoid space of rats. J Neurosci Methods. 90:81–86. 1999.
Article24. Moon Y, Glasgow WC, Eling TE. Curcumin suppresses interleukin 1beta-mediated microsomal prostaglandin E synthase 1 by altering early growth response gene 1 and other signaling pathways. J Pharmacol Exp Ther. 315:788–795. 2005.
Article25. Nakamura M, Okano H. Cell transplantation therapies for spinal cord injury focusing on induced pluripotent stem cells. Cell Res. 23:70–80. 2013.
Article26. Nicoll JA, Weller RO. A new role for astrocytes: beta-amyloid homeostasis and degradation. Trends Mol Med. 9:281–282. 2003.27. Sahin Kavaklı H, Koca C, Alıcı O. Antioxidant effects of curcumin in spinal cord injury in rats. Ulus Travma Acil Cerrahi Derg. 17:14–18. 2011.
Article28. Shehzad A, Lee YS. Molecular mechanisms of curcumin action: signal transd uction. Biofactors. 39:27–36. 2013.
Article29. Shibuya S, Miyamoto O, Auer RN, Itano T, Mori S, Norimatsu H. Embryonic intermediate filament, nestin, expression following traumatic spinal cord injury in adult rats. Neuroscience. 114:905–916. 2002.
Article30. Shih CH, Lacagnina M, Leuer-Bisciotti K, Pröschel C. Astroglial-derived periostin promotes axonal regeneration after spinal cord injury. J Neurosci. 34:2438–2443. 2014.
Article31. Shishodia S, Sethi G, Aggarwal BB. Curcumin: getting back to the roots. Ann N Y Acad Sci. 1056:206–217. 2005.
Article32. Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 5:146–156. 2004.
Article33. Soetikno V, Sari FR, Veeraveedu PT, Thandavarayan RA, Harima M, Sukumaran V, et al. Curcumin ameliorates macrophage infiltration by inhibiting NF-kB activation and proinflammatory cytokines in streptozotocin induced-diabetic nephropathy. Nutr Metab (Lond). 8:35. 2011.34. Somasundaram S, Edmund NA, Moore DT, Small GW, Shi YY, Orlowski RZ. Dietary curcumin inhibits chemotherapy-induced apoptosis in models of human breast cancer. Cancer Res. 62:3868–3875. 2002.35. Son S, Kim KT, Cho DC, Kim HJ, Sung JK, Bae JS. Curcumin stimulates proliferation of spinal cord neural progenitor cells via a mitogen-activated protein kinase signaling pathway. J Korean Neurosurg Soc. 56:1–4. 2014.
Article36. Suh HW, Kang S, Kwon KS. Curcumin attenuates glutamate-induced HT22 cell death by suppressing MAP kinase signaling. Mol Cell Biochem. 298:187–194. 2007.
Article37. Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 75:15–26. 1991.
Article38. Wells JE, Hurlbert RJ, Fehlings MG, Yong VW. Neuroprotection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain. 126(Pt 7):1628–1637. 2003.
Article39. Wu A, Ying Z, Gomez-Pinilla F. Dietary curcumin counteracts the outcome of traumatic brain injury on oxidative stress, synaptic plasticity, and cognition. Exp Neurol. 197:309–317. 2006.
Article40. Zhang X, Yin WK, Shi XD, Li Y. Curcumin activates Wnt/β-catenin signaling pathway through inhibiting the activity of GSK-3β in APPswe transfected SY5Y cells. Eur J Pharm Sci. 42:540–546. 2011.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Curcumin Stimulates Proliferation of Spinal Cord Neural Progenitor Cells via a Mitogen-Activated Protein Kinase Signaling Pathway
- Functional Recovery in Complete Spinal Cord Injury after Transplantation of Human Umbilical Cord Blood Cells in Rats
- Current Concept and Future of the Management of Spinal Cord Injury: A Systematic Review
- Neural Stem Cells
- Spinal Cord Regeneration in Rat using Neural Stem Cell Differentiated from Human Telencephalon