Cancer Res Treat.
2018 Jan;50(1):283-292. 10.4143/crt.2016.537.
Clinical Significance of Discordance between Carcinoembryonic Antigen Levels and RECIST in Metastatic Colorectal Cancer
- Affiliations
-
- 1Division of Medical Oncology, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. angelamd@catholic.ac.kr
- 2Department of Colorectal Cancer Centre, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Department of Surgery, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 4Department of Radiology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 5Cancer Research Institute, The Catholic University of Korea College of Medicine, Seoul, Korea.
- KMID: 2403497
- DOI: http://doi.org/10.4143/crt.2016.537
Abstract
- PURPOSE
The purpose of this study was to investigate the prognostic implications of carcinoembryonic antigen (CEA) levels that are inconsistent with Response Evaluation Criteria in Solid Tumor (RECIST) responses in metastatic colorectal cancer patients.
MATERIALS AND METHODS
We retrospectively evaluated 360 patients with at least one measurable lesion who received first-line palliative chemotherapy. CEA-response was defined as CEA-complete response (CR; CEA normalization), CEA-partial response (PR; ≥ 50% decrease in CEA levels), CEA-progressive disease (PD; ≥ 50% increase in CEA levels), and CEA-stable disease (SD; non-CR/PR/PD). Overall survival (OS) and progression-free survival (PFS) were evaluated according to CEA-response.
RESULTS
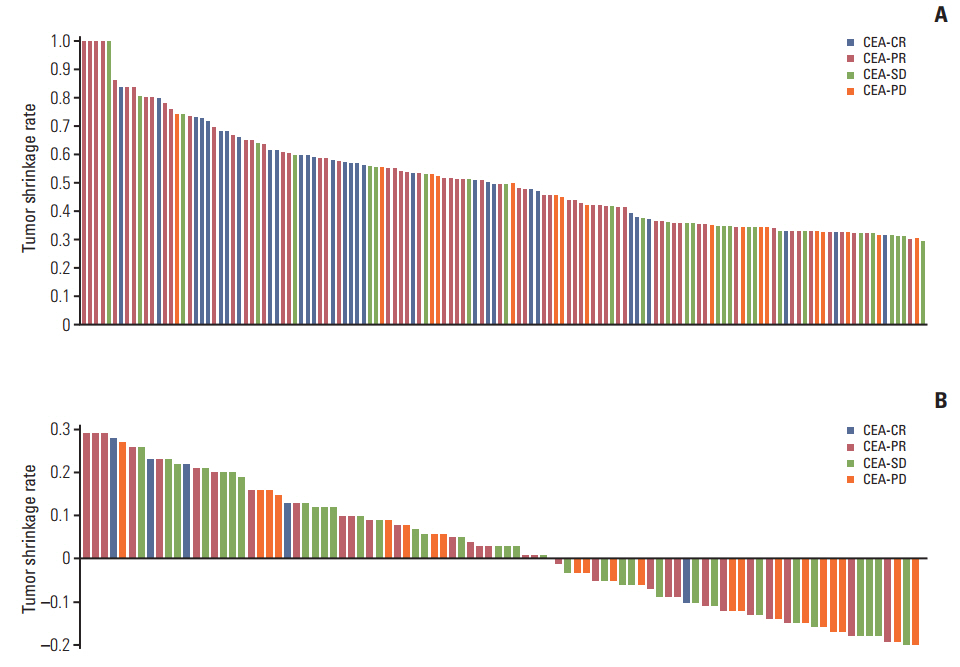
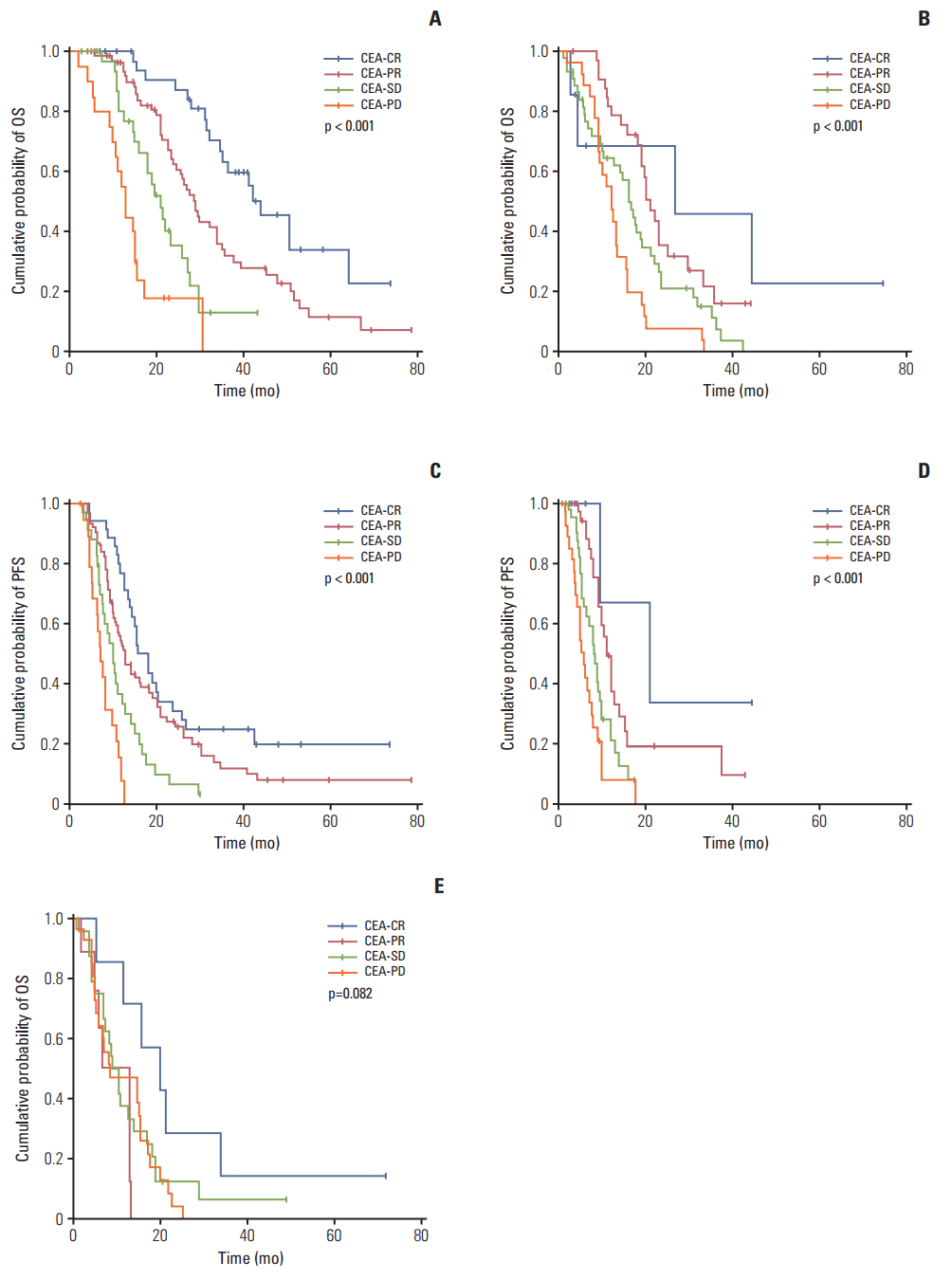
In RECIST-PR patients, poorer CEA-response was associated with disease progression at the subsequent evaluation. In RECIST-SD patients, CEA-CR and -PR were associated with lower disease progression rates than CEA-PD at the subsequent evaluation. Correlations between survival outcome and CEA-response in same-category RECIST patients were assessed. In RECIST-PR patients, discordant CEA-response (CEA-PD/SD) was associated with poorer survival than CEA-CR/PR (median OS and PFS, 44.0 and 15.4 [CEA-CR], 28.9 and 12.5 [CEA-PR], 21.0 and 9.8 [CEA-SD], and 13.0 and 7.0 [CEA-PD] months, respectively; all p < 0.001). In RECIST-SD patients, favorable CEA-response produced better survival (median OS and PFS, 26.8 and 21.0 [CEA-CR], 21.0 and 11.0 [CEA-PR], 16.1 and 8.2 [CEA-SD], and 12.2 and 6.0 [CEA-PD] months, respectively; all p < 0.001). RECIST-PD patients with CEA-CR showed longer OS than those with CEA-PD. Multivariate analysis demonstrated that discordant CEA-response is a powerful prognostic factor for RECIST-PR and RECIST-SD patients.
CONCLUSION
Among patients of the same RECIST-response categories, CEA-response patterns are significantly prognostic and strongly predictive of subsequent evaluation outcomes.
MeSH Terms
Figure
Cited by 1 articles
-
Tumor-Associated Macrophages Derived TGF-β‒Induced Epithelial to Mesenchymal Transition in Colorectal Cancer Cells through Smad2,3-4/Snail Signaling Pathway
Jianhui Cai, Limin Xia, Jinlei Li, Shichang Ni, Huayu Song, Xiangbin Wu
Cancer Res Treat. 2019;51(1):252-266. doi: 10.4143/crt.2017.613.
Reference
-
References
1. Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, et al. Colorectal cancer. Lancet. 2010; 375:1030–47.
Article2. Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004; 22:229–37.
Article3. Colucci G, Gebbia V, Paoletti G, Giuliani F, Caruso M, Gebbia N, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell'Italia Meridionale. J Clin Oncol. 2005; 23:4866–75.
Article4. Kirstein MM, Lange A, Prenzler A, Manns MP, Kubicka S, Vogel A. Targeted therapies in metastatic colorectal cancer: a systematic review and assessment of currently available data. Oncologist. 2014; 19:1156–68.
Article5. Kim G, Jung EJ, Ryu CG, Hwang DY. Usefulness of carcinoembryonic antigen for monitoring tumor progression during palliative chemotherapy in metastatic colorectal cancer. Yonsei Med J. 2013; 54:116–22.
Article6. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.
Article7. Huh JW, Oh BR, Kim HR, Kim YJ. Preoperative carcinoembryonic antigen level as an independent prognostic factor in potentially curative colon cancer. J Surg Oncol. 2010; 101:396–400.
Article8. Ward U, Primrose JN, Finan PJ, Perren TJ, Selby P, Purves DA, et al. The use of tumour markers CEA, CA-195 and CA-242 in evaluating the response to chemotherapy in patients with advanced colorectal cancer. Br J Cancer. 1993; 67:1132–5.
Article9. Preketes AP, King J, Caplehorn JR, Clingan PR, Ross WB, Morris DL. CEA reduction after cryotherapy for liver metastases from colon cancer predicts survival. Aust N Z J Surg. 1994; 64:612–4.
Article10. Mekenkamp LJ, Koopman M, Teerenstra S, van Krieken JH, Mol L, Nagtegaal ID, et al. Clinicopathological features and outcome in advanced colorectal cancer patients with synchronous vs metachronous metastases. Br J Cancer. 2010; 103:159–64.
Article11. Schwartz LH, Bogaerts J, Ford R, Shankar L, Therasse P, Gwyther S, et al. Evaluation of lymph nodes with RECIST 1.1. Eur J Cancer. 2009; 45:261–7.
Article12. Huang SC, Lin JK, Lin TC, Chen WS, Yang SH, Wang HS, et al. Concordance of Carcinoembryonic Antigen Ratio and Response Evaluation Criteria in Solid Tumors as Prognostic Surrogate Indicators of Metastatic Colorectal Cancer Patients Treated with Chemotherapy. Ann Surg Oncol. 2015; 22:2262–8.
Article13. Ruo L, Gougoutas C, Paty PB, Guillem JG, Cohen AM, Wong WD. Elective bowel resection for incurable stage IV colorectal cancer: prognostic variables for asymptomatic patients. J Am Coll Surg. 2003; 196:722–8.
Article14. Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005; 352:476–87.
Article15. Neki K, Kawahara H, Watanabe K, Toyama Y, Akiba T, Yanaga K. Usefulness of circulating tumor cells after preliminary chemotherapy for prediction of response to further anticancer therapy in patients with initially unresectable metastatic colorectal cancer. Anticancer Res. 2013; 33:1769–72.16. Zhao J, Li W, Zhu D, Yu Q, Zhang Z, Sun M, et al. Association of single nucleotide polymorphisms in MTHFR and ABCG2 with the different efficacy of first-line chemotherapy in metastatic colorectal cancer. Med Oncol. 2014; 31:802.
Article17. Hu J, Xu Y, Cai S. Specific microRNAs as novel biomarkers for combination chemotherapy resistance detection of colon adenocarcinoma. Eur J Med Res. 2015; 20:95.
Article18. Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007; 25:1753–9.
Article19. Adams RB, Aloia TA, Loyer E, Pawlik TM, Taouli B, Vauthey JN, et al. Selection for hepatic resection of colorectal liver metastases: expert consensus statement. HPB (Oxford). 2013; 15:91–103.
Article20. Loupakis F, Schirripa M, Caparello C, Funel N, Pollina L, Vasile E, et al. Histopathologic evaluation of liver metastases from colorectal cancer in patients treated with FOLFOXIRI plus bevacizumab. Br J Cancer. 2013; 108:2549–56.
Article21. Rubbia-Brandt L, Giostra E, Brezault C, Roth AD, Andres A, Audard V, et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007; 18:299–304.
Article22. Walker AS, Zwintscher NP, Johnson EK, Maykel JA, Stojadinovic A, Nissan A, et al. Future directions for monitoring treatment response in colorectal cancer. J Cancer. 2014; 5:44–57.
Article23. Cremolini C, Loupakis F, Antoniotti C, Lonardi S, Masi G, Salvatore L, et al. Early tumor shrinkage and depth of response predict long-term outcome in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab: results from phase III TRIBE trial by the Gruppo Oncologico del Nord Ovest. Ann Oncol. 2015; 26:1188–94.
Article24. Piessevaux H, Buyse M, Schlichting M, Van Cutsem E, Bokemeyer C, Heeger S, et al. Use of early tumor shrinkage to predict long-term outcome in metastatic colorectal cancer treated with cetuximab. J Clin Oncol. 2013; 31:3764–75.
Article25. Michl M, Stintzing S, Fischer von Weikersthal L, Decker T, Kiani A, Vehling-Kaiser U, et al. CEA response is associated with tumor response and survival in patients with KRAS exon 2 wild-type and extended RAS wild-type metastatic colorectal cancer receiving first-line FOLFIRI plus cetuximab or bevacizumab (FIRE-3 trial). Ann Oncol. 2016; 27:1565–72.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Serum Carcinoembryonic Antigen for Recurrence in Colorectal Cancer Patients
- Individualized Cutoff Value of the Serum Carcinoembryonic Antigen Level According to TNM Stage in Colorectal Cancer
- Changes of serum carcinoembryonic antigen in patients with colorectal cancer
- Functional role of carcinoembryonic antigen in the intercelluar adhesion of human colorectal carcinoma cell lines
- Comparison of WHO and RECIST Criteria for Response in Metastatic Colorectal Carcinoma