Cancer Res Treat.
2018 Jan;50(1):95-102. 10.4143/crt.2016.591.
Retrospective Molecular Epidemiology Study of PD-L1 Expression in Patients with EGFR-Mutant Non-small Cell Lung Cancer
- Affiliations
-
- 1Department of Thoracic Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. jhingookkim@gmail.com
- 2Merck & Co., Inc., Kenilworth, NJ, USA.
- 3Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 4Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2403478
- DOI: http://doi.org/10.4143/crt.2016.591
Abstract
- PURPOSE
Data are limited on programmed death ligand 1 (PD-L1) expression in epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer (NSCLC).
MATERIALS AND METHODS
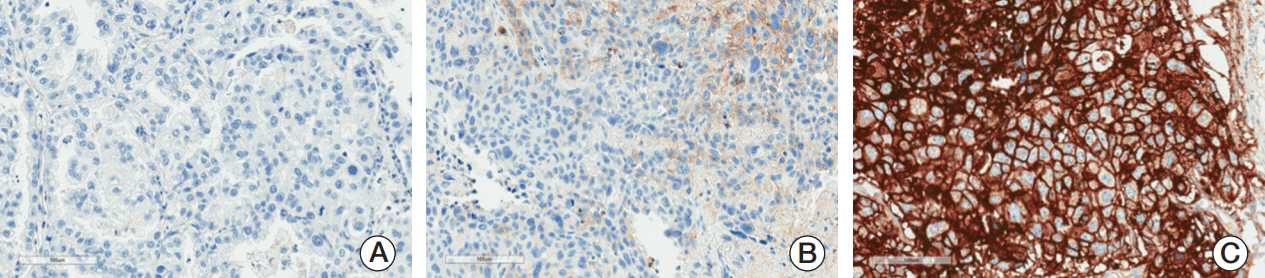
We retrospectively evaluated the relationship between PD-L1 expression and recurrence-free survival (RFS) and overall survival in 319 patients with EGFR-mutant NSCLC who were treated at Samsung Medical Center from 2006 to 2014. Membranous PD-L1 expression on tumor cells was measured using the PD-L1 IHC 22C3 pharmDx antibody and reported as tumor proportion score (TPS). Kaplan-Meier methods, log-rank test, and Cox proportional hazards models were used for survival analysis.
RESULTS
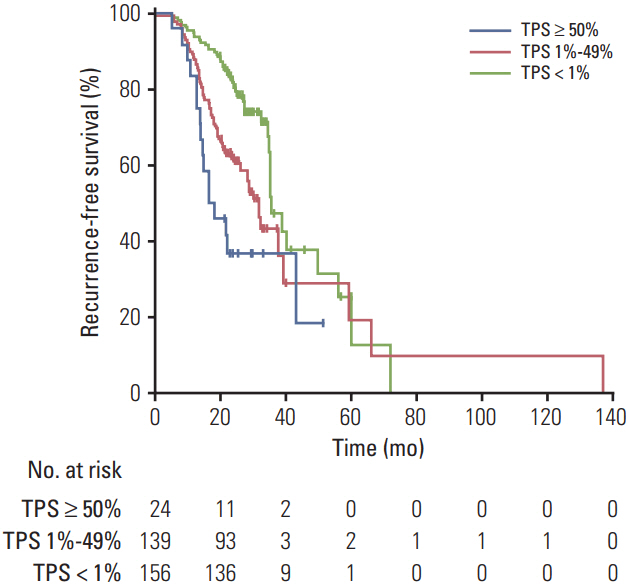
All patients had ≥1 EGFR mutation"”54% in exon 19 and 39% in exon 21. Overall, 51% of patients had PD-L1-positive tumors. The prevalence of PD-L1 positivity was higher among patients with stages II-IV versus stage I disease (64% vs. 44%) and among patients with other EGFR mutations (75%) than with L858R mutation (39%) or exon 19 deletion (52%). PD-L1 positivity was associated with shorter RFS, with an adjusted hazard ratio of 1.52 (95% confidence interval [CI], 0.81 to 2.84; median, 18 months) for the PD-L1 TPS ≥ 50% group, 1.51 (95% CI, 1.02 to 2.21; median, 31 months) for the PD-L1 TPS 1%-49% group, and 1.51 (95% CI, 1.05 to 2.18) for the combined PD-L1-positive groups (TPS ≥ 1%) compared with the PD-L1-negative group (median, 35 months).
CONCLUSION
PD-L1 expression is associated with disease stage and type of EGFR mutation. PD-L1 positivity might be associated with worse RFS among patients with surgically treated EGFR-mutant NSCLC.
Keyword
MeSH Terms
Figure
Reference
-
References
1. D’Addario G, Fruh M, Reck M, Baumann P, Klepetko W, Felip E, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010; 21 Suppl 5:v116–9.2. Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005; 97:339–46.
Article3. Bronte G, Rizzo S, La Paglia L, Adamo V, Siragusa S, Ficorella C, et al. Driver mutations and differential sensitivity to targeted therapies: a new approach to the treatment of lung adenocarcinoma. Cancer Treat Rev. 2010; 36 Suppl 3:S21–9.
Article4. Luo SY, Lam DC. Oncogenic driver mutations in lung cancer. Transl Respir Med. 2013; 1:6.
Article5. Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014; 9:154–62.
Article6. McDermott DF, Atkins MB. PD-1 as a potential target in cancer therapy. Cancer Med. 2013; 2:662–73.7. D’Incecco A, Andreozzi M, Ludovini V, Rossi E, Capodanno A, Landi L, et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer. 2015; 112:95–102.
Article8. Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008; 8:467–77.
Article9. Sun JM, Zhou W, Choi YL, Choi SJ, Kim SE, Wang Z, et al. Prognostic significance of PD-L1 in patients with non-small cell lung cancer: a large cohort study of surgically resected cases. J Thorac Oncol. 2016; 11:1003–11.
Article10. Azuma K, Ota K, Kawahara A, Hattori S, Iwama E, Harada T, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol. 2014; 25:1935–40.
Article11. Sul J, Blumenthal GM, Jiang X, He K, Keegan P, Pazdur R. FDA approval summary: pembrolizumab for the treatment of patients with metastatic non-small cell lung cancer whose tumors express programmed death-ligand 1. Oncologist. 2016; 21:643–50.
Article12. Kazandjian D, Suzman DL, Blumenthal G, Mushti S, He K, Libeg M, et al. FDA approval summary: nivolumab for the treatment of metastatic non-small cell lung cancer with progression on or after platinum-based chemotherapy. Oncologist. 2016; 21:634–42.
Article13. Genentech, Inc. Tecentriq [package insert]. South San Fransisco, CA: Genentech, Inc.;2016.14. Boland JM, Kwon ED, Harrington SM, Wampfler JA, Tang H, Yang P, et al. Tumor B7-H1 and B7-H3 expression in squamous cell carcinoma of the lung. Clin Lung Cancer. 2013; 14:157–63.
Article15. Chen YB, Mu CY, Huang JA. Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: a 5-year-follow-up study. Tumori. 2012; 98:751–5.
Article16. Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. 2011; 28:682–8.
Article17. Yang CY, Lin MW, Chang YL, Wu CT, Yang PC. Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer. 2014; 50:1361–9.
Article18. Dolled-Filhart M, Roach C, Toland G, Stanforth D, Jansson M, Lubiniecki GM, et al. Development of a companion diagnostic for pembrolizumab in non-small cell lung cancer using immunohistochemistry for programmed death ligand-1. Arch Pathol Lab Med. 2016; Aug. 23. [Epub]. https://doi.org/10.5858/arpa.2015-0542-OA.
Article19. Roach C, Zhang N, Corigliano E, Jansson M, Toland G, Ponto G, et al. Development of a companion diagnostic PD-L1 immunohistochemistry assay for pembrolizumab therapy in non-small-cell lung cancer. Appl Immunohistochem Mol Morphol. 2016; 24:392–7.
Article20. Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer-Verlag;2010.21. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015; 372:2018–28.
Article22. Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016; 387:1540–50.
Article23. Sun JM, Zhou W, Choi YL, Choi SJ, Kim SE, Wang Z, et al. PD-L1 expression and survival in patients with non-small cell lung cancer (NSCLC) in Korea. J Clin Oncol. 2014; 32 Suppl 5:Abstr 8066.
Article24. Ji M, Liu Y, Li Q, Li X, Ning Z, Zhao W, et al. PD-1/PD-L1 expression in non-small-cell lung cancer and its correlation with EGFR/KRAS mutations. Cancer Biol Ther. 2016; 17:407–13.
Article25. Ji M, Liu Y, Li Q, Li XD, Zhao WQ, Zhang H, et al. PD-1/PD-L1 pathway in non-small-cell lung cancer and its relation with EGFR mutation. J Transl Med. 2015; 13:5.
Article26. Tang Y, Fang W, Zhang Y, Hong S, Kang S, Yan Y, et al. The association between PD-L1 and EGFR status and the prognostic value of PD-L1 in advanced non-small cell lung cancer patients treated with EGFR-TKIs. Oncotarget. 2015; 6:14209–19.
Article27. Lin K, Cheng J, Yang T, Li Y, Zhu B. EGFR-TKI down-regulates PD-L1 in EGFR mutant NSCLC through inhibiting NF-κB. Biochem Biophys Res Commun. 2015; 463:95–101.
Article28. Rizvi NA, Chow LQ, Borghaei H, Shen Y, Harbison C, Alaparthy S, et al. Safety and response with nivolumab (anti-PD-1; BMS-936558, ONO-4538) plus erlotinib in patients (pts) with epidermal growth factor receptor mutant (EGFR MT) advanced NSCLC. J Clin Oncol. 2014; 32 Suppl 5:Abstr 8022.
Article29. Sorensen SF, Zhou W, Dolled-Filhart M, Georgsen JB, Wang Z, Emancipator K, et al. PD-L1 expression and survival among patients with advanced non-small cell lung cancer treated with chemotherapy. Transl Oncol. 2016; 9:64–9.
Article30. Schmidt LH, Kummel A, Gorlich D, Mohr M, Brockling S, Mikesch JH, et al. PD-1 and PD-L1 expression in NSCLC indicate a favorable prognosis in defined subgroups. PLoS One. 2015; 10:e0136023.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlation of PD-L1 Expression Tested by 22C3 and SP263 in Non-Small Cell Lung Cancer and Its Prognostic Effect on EGFR Mutation–Positive Lung Adenocarcinoma
- PD-L1 Testing in Non-small Cell Lung Cancer: Past, Present, and Future
- Molecularly Targeted Therapy for Lung Cancer : Recent Topics
- Programmed death-ligand 1 expression and tumor-infiltrating lymphocytes in non-small cell lung cancer: association with clinicopathologic parameters
- Temporal evolution of programmed death-ligand 1 expression in patients with non-small cell lung cancer