Tuberc Respir Dis.
2020 Jan;83(1):51-60. 10.4046/trd.2019.0026.
Correlation of PD-L1 Expression Tested by 22C3 and SP263 in Non-Small Cell Lung Cancer and Its Prognostic Effect on EGFR Mutation–Positive Lung Adenocarcinoma
- Affiliations
-
- 1Department of Internal Medicine, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. yschang@yuhs.ac
- 2Department of Pathology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2466620
- DOI: http://doi.org/10.4046/trd.2019.0026
Abstract
- BACKGROUND
Programmed death-ligand 1 (PD-L1) expression is tested by immunohistochemistry (IHC)"”22C3, SP263, and SP142. The aim of this study is to evaluate the correlation among the three methods of PD-L1 IHC in non-small cell lung cancer (NSCLC) and clinical significance of PD-L1 expression in lung adenocarcinoma with an epidermal growth factor receptor (EGFR)-tyrosine kinase domain mutation.
METHODS
The results of 230 patients who were pathologically confirmed as having NSCLC; tested using PD-L1 IHC 22C3, SP263, and SP142 methods; and evaluated via the peptide nucleic acid clamping method to confirm EGFR mutation, were analyzed in this study.
RESULTS
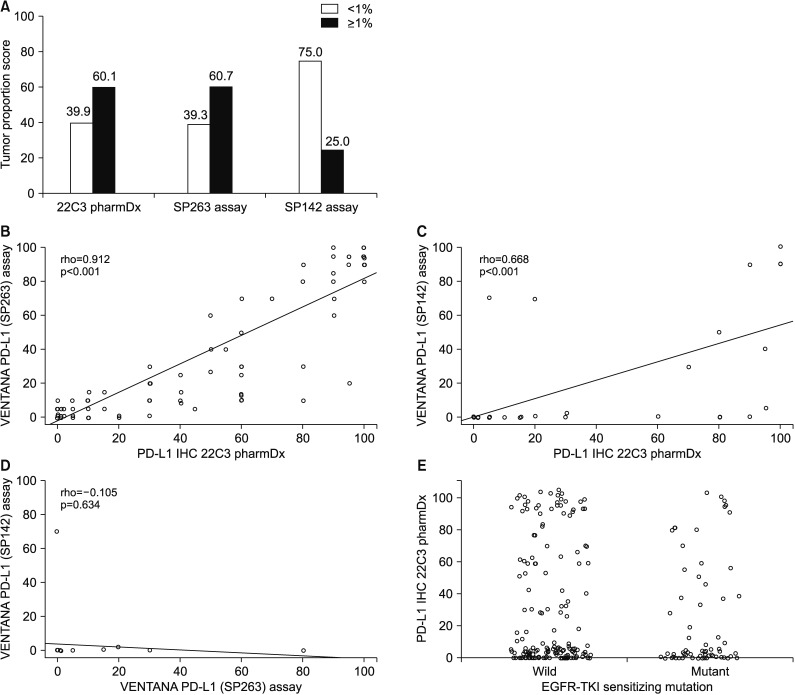
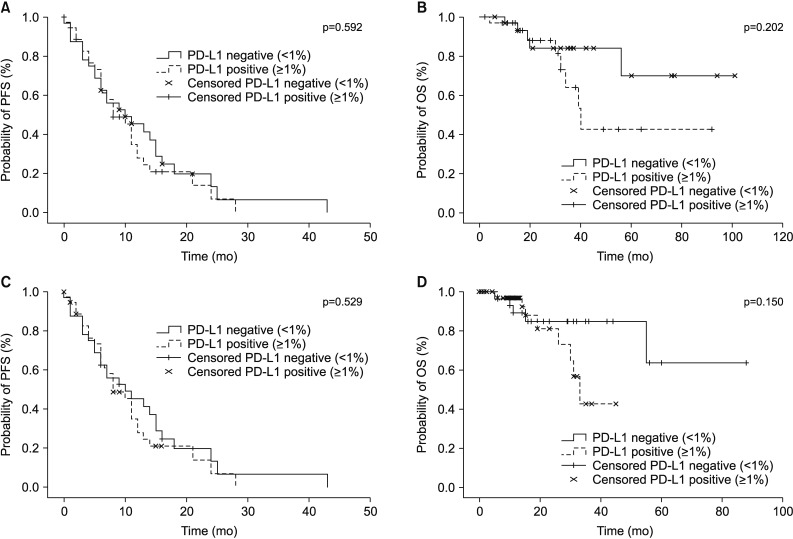
164 patients underwent both the SP263 and 22C3 tests. There was a significant positive correlation between the outcomes of the two tests (Spearman correlation coefficient=0.912, p<0.001), with a derived regression equation as follows: 22C3=15.2+0.884×SP263 (R2=0.792, p<0.001). There was no relationship between the expression of PD-L1 and clinical parameters, including EGFR-tyrosine kinase inhibitor (TKI) mutation. The PD-L1 expression in patients treated with EGFR-TKI yielded a 2-month-shorter progression period than that in the PD-L1-negative group. However, this did not reach statistical significance (PD-L1<1% vs. PD-L1≥1%, 10 months vs. 8 months).
CONCLUSION
The results of the 22C3 and those of SP263 methods were in good correlation with one another. Since the PD-L1 expression is not influenced by the EGFR mutation, it is necessary to perform a PD-L1 test to set the treatment direction in the patients with EGFR-mutant NSCLC.
Keyword
MeSH Terms
Figure
Reference
-
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424. PMID: 30207593.
Article2. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015; 373:1627–1639. PMID: 26412456.
Article3. Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015; 373:123–135. PMID: 26028407.
Article4. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015; 372:2018–2028. PMID: 25891174.
Article5. Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015; 372:320–330. PMID: 25399552.6. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015; 373:23–34. PMID: 26027431.
Article7. Goss G, Tsai CM, Shepherd FA, Bazhenova L, Lee JS, Chang GC, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2016; 17:1643–1652. PMID: 27751847.
Article8. Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology: mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015; 348:124–128. PMID: 25765070.9. Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016; 387:1540–1550. PMID: 26712084.
Article10. Rizvi NA, Mazieres J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015; 16:257–265. PMID: 25704439.
Article11. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016; 387:1837–1846. PMID: 26970723.
Article12. Hirsch FR, McElhinny A, Stanforth D, Ranger-Moore J, Jansson M, Kulangara K, et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the Blueprint PD-L1 IHC assay comparison project. J Thorac Oncol. 2017; 12:208–222. PMID: 27913228.
Article13. Marchetti A, Barberis M, Franco R, De Luca G, Pace MV, Staibano S, et al. Multicenter Comparison of 22C3 PharmDx (Agilent) and SP263 (Ventana) assays to test PD-L1 expression for NSCLC patients to be treated with immune checkpoint inhibitors. J Thorac Oncol. 2017; 12:1654–1663. PMID: 28818609.
Article14. Roach C, Zhang N, Corigliano E, Jansson M, Toland G, Ponto G, et al. Development of a companion diagnostic PD-L1 immunohistochemistry assay for pembrolizumab therapy in non-small-cell lung cancer. Appl Immunohistochem Mol Morphol. 2016; 24:392–397. PMID: 27333219.
Article15. Kim HJ, Lee KY, Kim YC, Kim KS, Lee SY, Jang TW, et al. Detection and comparison of peptide nucleic acid-mediated real-time polymerase chain reaction clamping and direct gene sequencing for epidermal growth factor receptor mutations in patients with non-small cell lung cancer. Lung Cancer. 2012; 75:321–325. PMID: 21930325.
Article16. Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005; 97:339–346. PMID: 15741570.
Article17. Offin M, Rizvi H, Tenet M, Ni A, Sanchez-Vega F, Li BT, et al. Tumor mutation burden and efficacy of EGFR-tyrosine kinase inhibitors in patients with EGFR-mutant lung cancers. Clin Cancer Res. 2019; 25:1063–1069. PMID: 30045933.
Article18. Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. 2011; 28:682–688. PMID: 20373055.
Article19. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016; 375:1823–1833. PMID: 27718847.
Article20. Azuma K, Ota K, Kawahara A, Hattori S, Iwama E, Harada T, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol. 2014; 25:1935–1940. PMID: 25009014.
Article21. Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013; 3:1355–1363. PMID: 24078774.
Article22. Chen N, Fang W, Zhan J, Hong S, Tang Y, Kang S, et al. Upregulation of PD-L1 by EGFR activation mediates the immune escape in EGFR-driven NSCLC: implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J Thorac Oncol. 2015; 10:910–923. PMID: 25658629.
Article23. Tang Y, Fang W, Zhang Y, Hong S, Kang S, Yan Y, et al. The association between PD-L1 and EGFR status and the prognostic value of PD-L1 in advanced non-small cell lung cancer patients treated with EGFR-TKIs. Oncotarget. 2015; 6:14209–14219. PMID: 25895031.
Article24. Zhang Y, Wang L, Li Y, Pan Y, Wang R, Hu H, et al. Protein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocarcinoma. Onco Targets Ther. 2014; 7:567–573. PMID: 24748806.
Article25. Ji M, Liu Y, Li Q, Li X, Ning Z, Zhao W, et al. PD-1/PD-L1 expression in non-small-cell lung cancer and its correlation with EGFR/KRAS mutations. Cancer Biol Ther. 2016; 17:407–413. PMID: 26954523.
Article26. Lin C, Chen X, Li M, Liu J, Qi X, Yang W, et al. Programmed death-ligand 1 expression predicts tyrosine kinase inhibitor response and better prognosis in a cohort of patients with epidermal growth factor receptor mutation-positive lung adenocarcinoma. Clin Lung Cancer. 2015; 16:e25–e35. PMID: 25801750.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Characteristics of Korean Patients with Lung Cancer Who Have Programmed Death-Ligand 1 Expression
- Concordance of Programmed DeathLigand 1 Expression between SP142 and 22C3/SP263 Assays in Triple-Negative Breast Cancer
- Programmed death-ligand 1 expression and tumor-infiltrating lymphocytes in non-small cell lung cancer: association with clinicopathologic parameters
- Human Leukocyte Antigen Class I and Programmed Death-Ligand 1 Coexpression Is an Independent Poor Prognostic Factor in Adenocarcinoma of the Lung
- PD-L1 Testing in Gastric Cancer by the Combined Positive Score of the 22C3 PharmDx and SP263 Assay with Clinically Relevant Cut-offs