Nat Prod Sci.
2017 Dec;23(4):239-246. 10.20307/nps.2017.23.4.239.
Inhibitory Effects of Methanol Extract from Nardostachys chinensis on 27-hydroxycholesterol-induced Differentiation of Monocytic Cells
- Affiliations
-
- 1Department of Pharmacology, School of Medicine, Pusan National University, Yangsan 50612, Republic of Korea. koanhoi@pusan.ac.kr
- 2Division of Pharmacology, School of Korean Medicine, Pusan National University, Yangsan 50612, Republic of Korea.
- 3Department of Microbiology and Immunology, Pusan National University - School of Medicine, Yangsan, Gyeongnam 50612, Republic of Korea.
- 4Institute of Marine BioTechnology, Pusan National University, Busan, Republic of Korea.
- KMID: 2401574
- DOI: http://doi.org/10.20307/nps.2017.23.4.239
Abstract
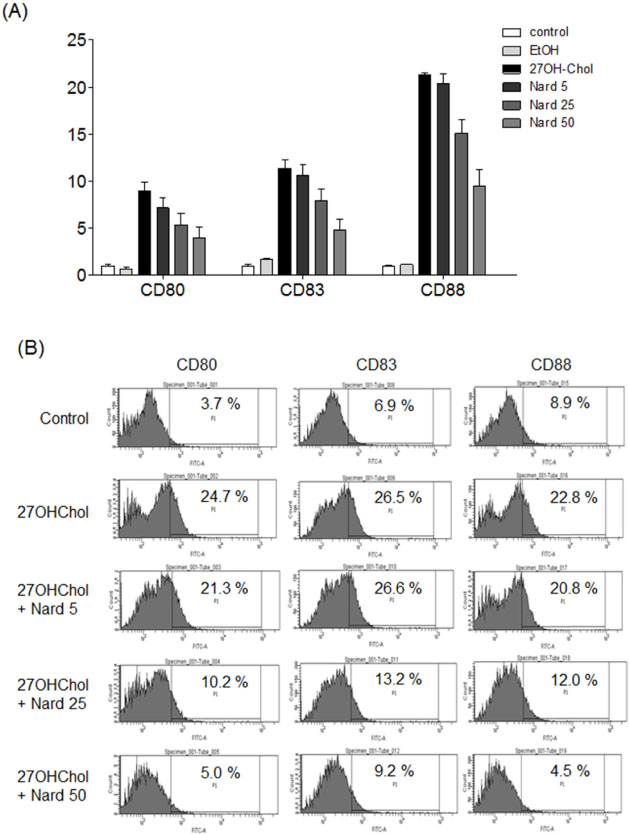
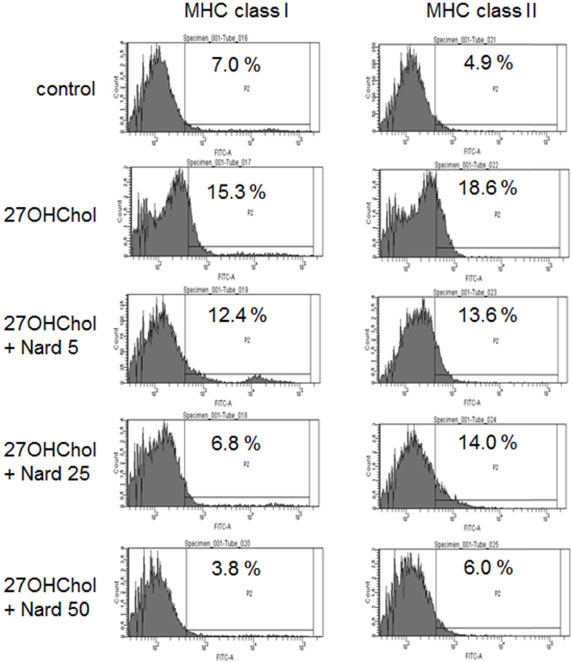
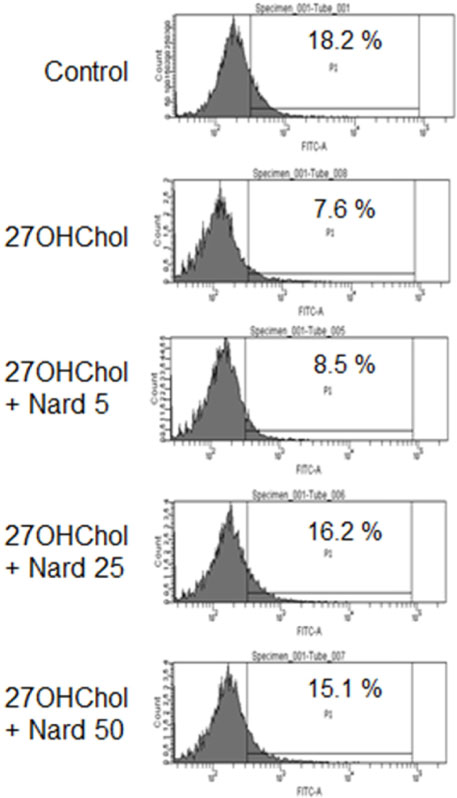
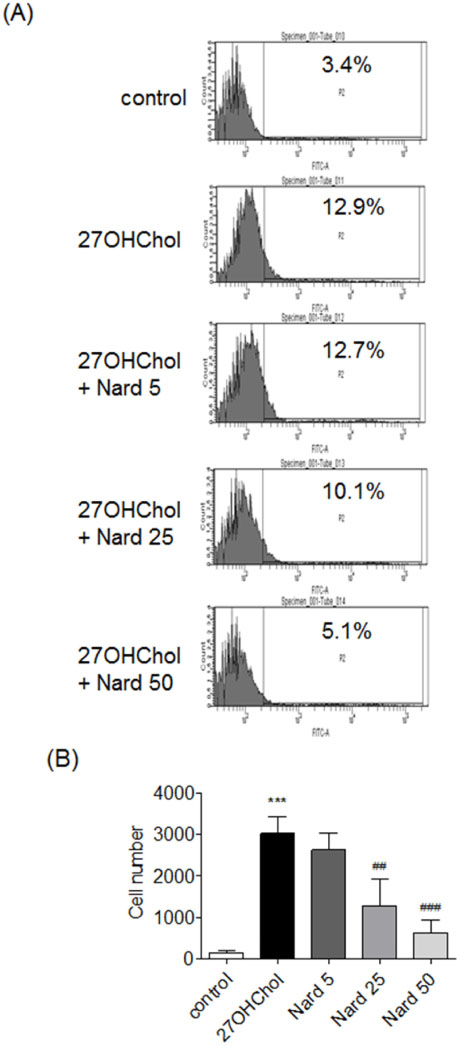
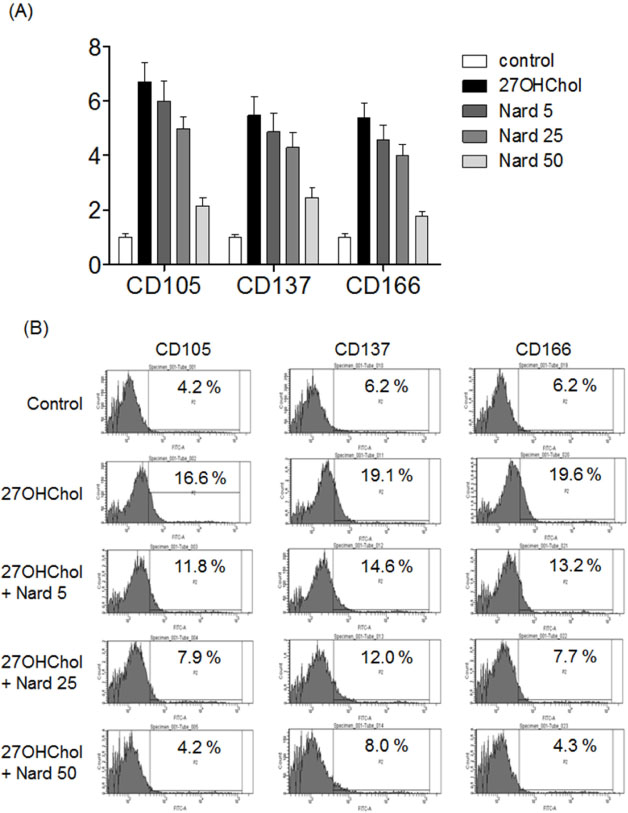
- 27-Hydroxycholesterol (27OHChol) has been reported to induce differentiation of monocytic cells into a mature dendritic cell phenotype. We examined the effect of methanol extract of Nardostachys chinensis (Nard) on 27OHChol-induced differentiation using THP-1, a human monocytic cell line. Treatment of monocytic cells with methanol extract of Nard resulted in decreased transcription and surface expression of CD80, CD83, and CD88 elevated by 27OHChol in a dose-dependent manner. Surface levels of MHC class I and II molecules elevated by 27OHChol were also reduced to basal levels by treatment with the Nard extract. Decreased endocytosis activity caused by 27OHChol was recovered by treatment with the Nard extract. CD197 expression and cell attachment were attenuated by the Nard extract. In addition, levels of transcription and surface expression of CD molecules involved in atherosclerosis, such as CD105, CD137, and CD166 upregulated by 27OHChol were significantly decreased by treatment with methanol extract of Nard. These results indicate that methanol extract of Nard down-regulates 27OHChol-induced differentiation of monocytic cells into a mature dendritic cell phenotype and expression of CD molecules associated with atherosclerosis. The current study suggests that biological activity of oxygenated cholesterol derivatives can be inhibited by herbal medication.
MeSH Terms
Figure
Reference
-
1. Sallusto F, Lanzavecchia A. J Exp Med. 1994; 179:1109–1118.2. Zhou LJ, Tedder TF. Proc Natl Acad Sci U S A. 1996; 93:2588–2592.3. Lyakh LA, Koski GK, Telford W, Gress RE, Cohen PA, Rice NR. J Immunol. 2000; 165:3647–3655.4. Berges C, Naujokat C, Tinapp S, Wieczorek H, Höh A, Sadeghi M, Opelz G, Daniel V. Biochem Biophys Res Commun. 2005; 333:896–907.5. Son Y, Kim SM, Lee SA, Eo SK, Kim K. Biochem Biophys Res Commun. 2013; 438:161–168.6. Takaya Y, Takeuji Y, Akasaka M, Nakagawasai O, Tadano T, Kisara K, Kim K, Wataya Y, Niwa M, Oshima Y. Tetrahedron. 2000; 56:7673–7678.7. Zhang J, Qiang CC, Li WJ, Liu LJ, Lin XX, Cheng YJ, Tang K, Yao FJ, Wu SH. J Cardiovasc Pharmacol. 2014; 64:127–133.8. Takemoto H, Omameuda Y, Ito M, Fukuda T, Kaneko S, Akaike A, Kobayashi Y. Biol Pharm Bull. 2014; 37:1050–1055.9. Hwang JS, Lee SA, Hong SS, Han XH, Lee C, Lee D, Lee CK, Hong JT, Kim Y, Lee MK, Hwang BY. Bioorg Med Chem Lett. 2012; 22:706–708.10. Seo HC, Kim SM, Eo SK, Rhim BY, Kim K. Biomol Ther (Seoul). 2015; 23:84–89.11. Steinman RM. Annu Rev Immunol. 1991; 9:271–296.12. Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Annu Rev Immunol. 2000; 18:767–811.13. Perry HM, McNamara CA. Arterioscler Thromb Vasc Biol. 2012; 32:1548–1549.14. Profumo E, Buttari B, Saso L, Capoano R, Salvati B, Riganò R. Scientific World J. 2012; 2012:157534.15. Katz FE, Parkar M, Stanley K, Murray LJ, Clark EA, Greaves MF. Eur J Immunol. 1985; 15:103–106.16. Fujimoto Y, Tu L, Miller AS, Bock C, Fujimoto M, Doyle C, Steeber DA, Tedder TF. Cell. 2002; 108:755–767.17. Gavett SH, O'Hearn DJ, Li X, Huang SK, Finkelman FD, Wills-Karp M. J Exp Med. 1995; 182:1527–1536.18. Banchereau J, Steinman RM. Nature. 1998; 392:245–252.19. Moschovakis GL, Bubke A, Dittrich-Breiholz O, Braun A, Prinz I, Kremmer E, Förster R. Eur J Immunol. 2012; 42:48–57.20. DuSell CD, Umetani M, Shaul PW, Mangelsdorf DJ, McDonnell DP. Mol Endocrinol. 2008; 22:65–77.21. Piao M, Tokunaga O. J Atheroscler Thromb. 2006; 13:82–89.22. Olofsson PS, Söderström LA, Wågsäter D, Sheikine Y, Ocaya P, Lang F, Rabu C, Chen L, Rudling M, Aukrust P, Hedin U, Paulsson-Berne G, Sirsjö A, Hansson GK. Circulation. 2008; 117:1292–1301.23. Kim SM, Kim BY, Lee SA, Eo SK, Yun Y, Kim CD, Kim K. Toxicol Appl Pharmacol. 2014; 274:462–470.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diclofenac Inhibits 27-hydroxycholesterol-induced Differentiation of Monocytic Cells into Mature Dendritic Cells
- PI3K and ERK signaling pathways are involved in differentiation of monocytic cells induced by 27-hydroxycholesterol
- Inhibitory effects of Coptis chinensis extract on the growth and biofilm formation of Streptococcus mutans and Streptococcus sobrinus
- Studies on anticancer effects of extracts caesalpinia sappan on oral carcinoma and osteosarcoma cells
- Lignans with NADPH Oxidase 2 (NOX2)-inhibitory Activity from the Fruits of Schisandra chinensis