Korean J Physiol Pharmacol.
2017 May;21(3):301-308. 10.4196/kjpp.2017.21.3.301.
PI3K and ERK signaling pathways are involved in differentiation of monocytic cells induced by 27-hydroxycholesterol
- Affiliations
-
- 1Department of Pharmacology, Pusan National University School of Medicine, Yangsan 50612, Korea. koanhoi@pusan.ac.kr
- 2Department of Microbiology & Immunology, Pusan National University School of Medicine, Yangsan 50612, Korea.
- 3College of Veterinary Medicine and Bio-Safety Research Institute, Chonbuk National University, Iksan 54596, Korea.
- 4Institute of Marine BioTechnology, Pusan National University, Busan 46241, Korea.
- 5Department of Neurosurgery, Kosin University College of Medicine, Busan 49267, Korea.
- KMID: 2376958
- DOI: http://doi.org/10.4196/kjpp.2017.21.3.301
Abstract
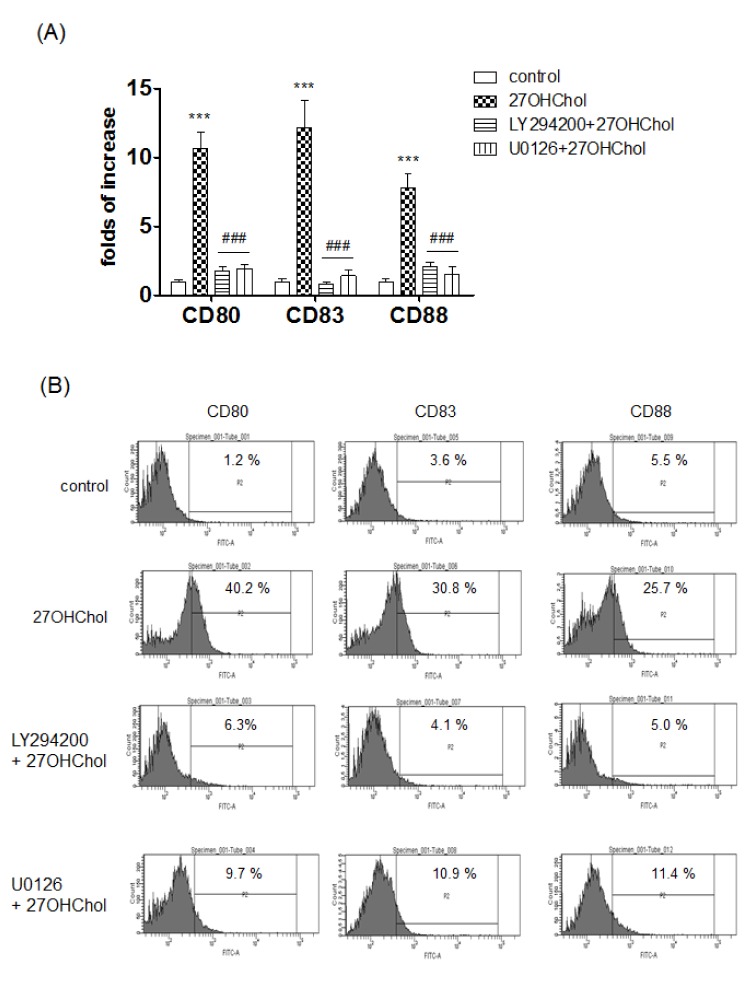
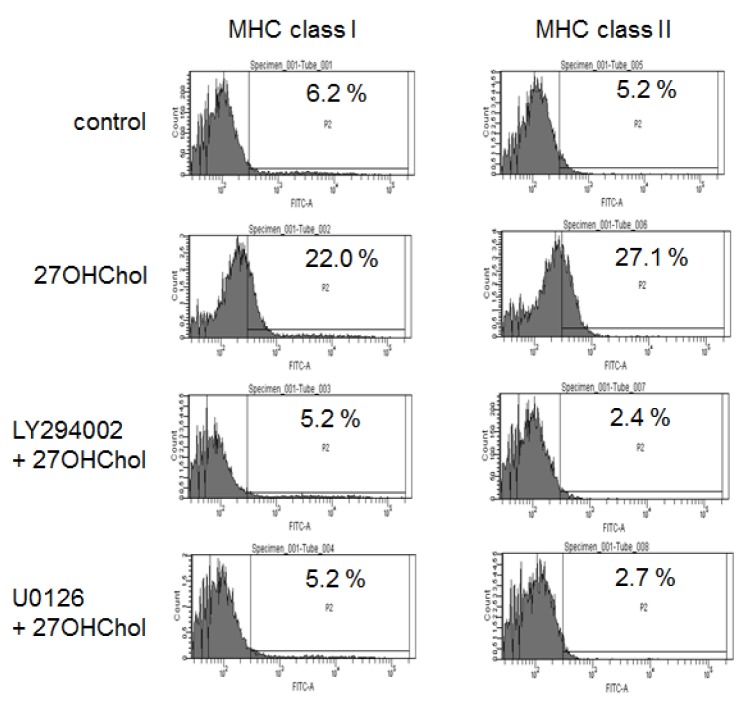
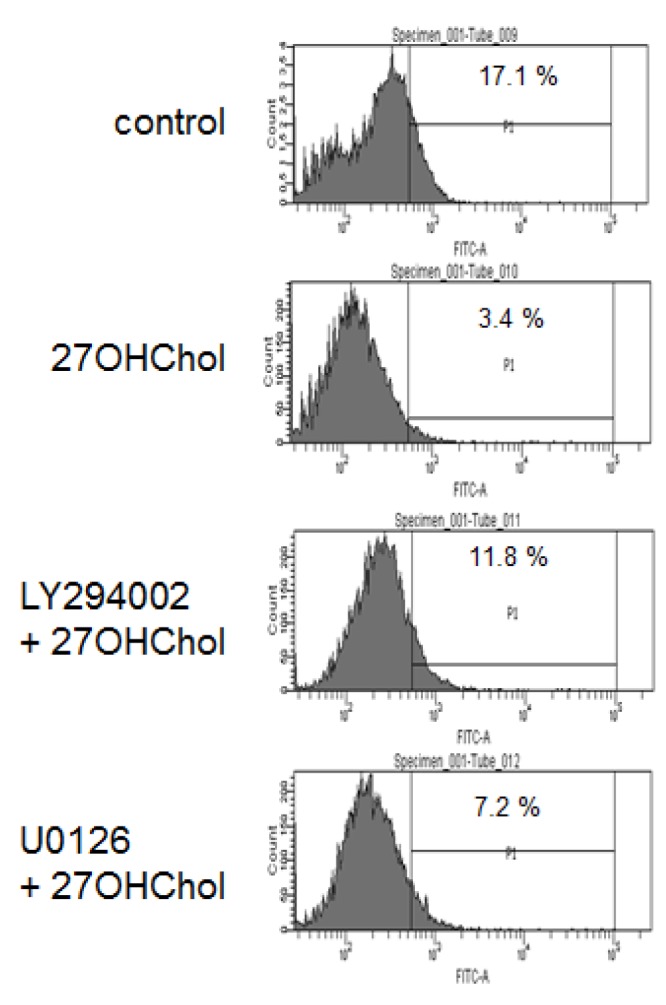
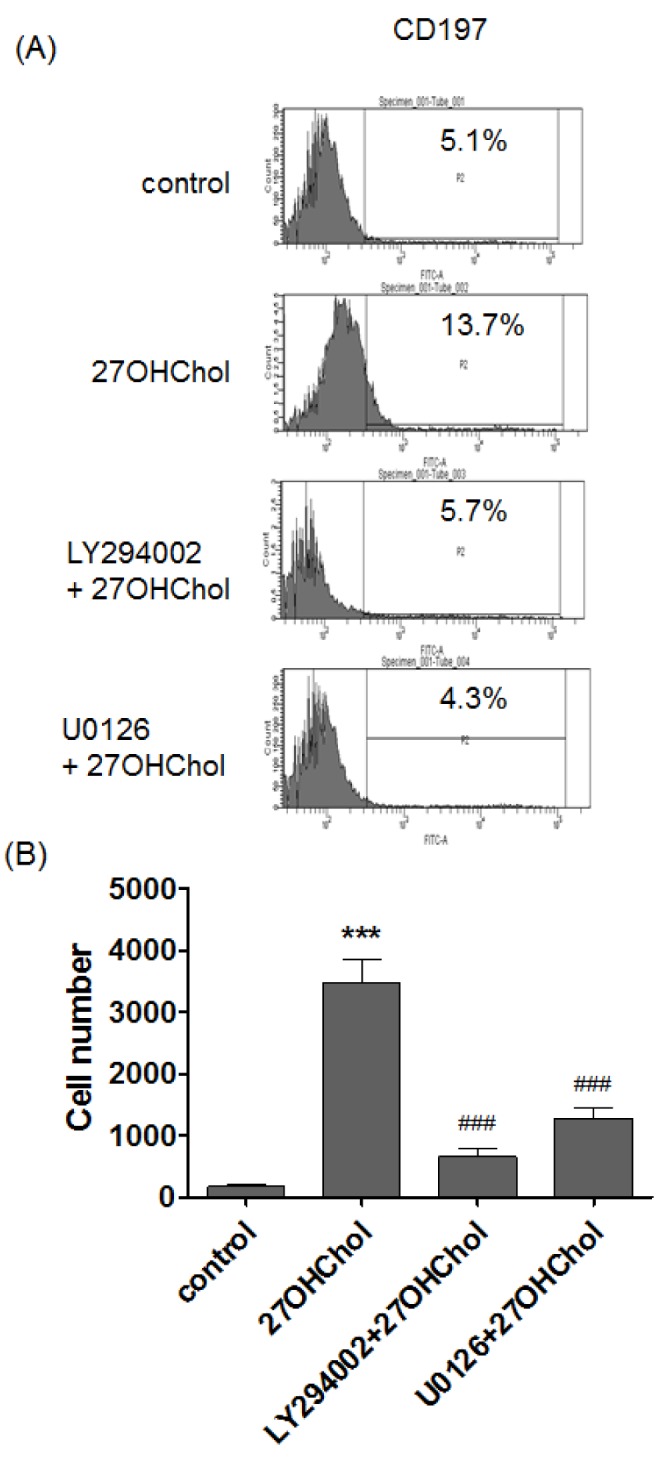
- 27-Hydroxycholesterol induces differentiation of monocytic cells into mature dendritic cells, mDCs. In the current study we sought to determine roles of the PI3K and the ERK pathways in the 27OHChol-induced differentiation. Up-regulation of mDC-specific markers like CD80, CD83 and CD88 induced by stimulation with 27OHChol was significantly reduced in the presence of LY294002, an inhibitor of PI3K, and U0126, an inhibitor of ERK. Surface expression of MHC class I and II molecules elevated by 27OHChol was decreased to basal levels in the presence of the inhibitors. Treatment with LY294002 or U0126 resulted in recovery of endocytic activity which was reduced by 27OHChol. CD197 expression and cell adherence enhanced by 27OHChol were attenuated in the presence of the inhibitors. Transcription and surface expression of CD molecules involved in atherosclerosis such as CD105, CD137 and CD166 were also significantly decreased by treatment with LY294002 and U0126. These results mean that the PI3K and the ERK signaling pathways are necessary for differentiation of monocytic cells into mDCs and involved in over-expression of atherosclerosis-associated molecules in response to 27OHChol.
Keyword
Figure
Cited by 1 articles
-
The role of 27-hydroxycholesterol in meta-inflammation
Yonghae Son, Eunbeen Choi, Yujin Hwang, Koanhoi Kim
Korean J Physiol Pharmacol. 2024;28(2):107-112. doi: 10.4196/kjpp.2024.28.2.107.
Reference
-
1. Brown AJ, Jessup W. Oxysterols and atherosclerosis. Atherosclerosis. 1999; 142:1–28. PMID: 9920502.
Article2. Berliner JA, Navab M, Fogelman AM, Frank JS, Demer LL, Edwards PA, Watson AD, Lusis AJ. Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation. 1995; 91:2488–2496. PMID: 7729036.3. Kim SM, Kim BY, Lee SA, Eo SK, Yun Y, Kim CD, Kim K. 27-Hydroxycholesterol and 7alpha-hydroxycholesterol trigger a sequence of events leading to migration of CCR5-expressing Th1 lymphocytes. Toxicol Appl Pharmacol. 2014; 274:462–470. PMID: 24370436.
Article4. Kim SM, Lee SA, Kim BY, Bae SS, Eo SK, Kim K. 27-Hydroxycholesterol induces recruitment of monocytic cells by enhancing CCL2 production. Biochem Biophys Res Commun. 2013; 442:159–164. PMID: 24269812.
Article5. Son Y, Kim SM, Lee SA, Eo SK, Kim K. Oxysterols induce transition of monocytic cells to phenotypically mature dendritic cell-like cells. Biochem Biophys Res Commun. 2013; 438:161–168. PMID: 23876312.
Article6. Kim SM, Kim BY, Eo SK, Kim CD, Kim K. 27-Hydroxycholesterol up-regulates CD14 and predisposes monocytic cells to superproduction of CCL2 in response to lipopolysaccharide. Biochim Biophys Acta. 2015; 1852:442–450. PMID: 25497142.
Article7. Umetani M, Ghosh P, Ishikawa T, Umetani J, Ahmed M, Mineo C, Shaul PW. The cholesterol metabolite 27-hydroxycholesterol promotes atherosclerosis via proinflammatory processes mediated by estrogen receptor alpha. Cell Metab. 2014; 20:172–182. PMID: 24954418.
Article8. Wu Q, Ishikawa T, Sirianni R, Tang H, McDonald JG, Yuhanna IS, Thompson B, Girard L, Mineo C, Brekken RA, Umetani M, Euhus DM, Xie Y, Shaul PW. 27-Hydroxycholesterol promotes cell-autonomous, ER-positive breast cancer growth. Cell Rep. 2013; 5:637–645. PMID: 24210818.
Article9. Vurusaner B, Gamba P, Testa G, Gargiulo S, Biasi F, Zerbinati C, Iuliano L, Leonarduzzi G, Basaga H, Poli G. Survival signaling elicited by 27-hydroxycholesterol through the combined modulation of cellular redox state and ERK/Akt phosphorylation. Free Radic Biol Med. 2014; 77:376–385. PMID: 25110320.
Article10. Vurusaner B, Gamba P, Gargiulo S, Testa G, Staurenghi E, Leonarduzzi G, Poli G, Basaga H. Nrf2 antioxidant defense is involved in survival signaling elicited by 27-hydroxycholesterol in human promonocytic cells. Free Radic Biol Med. 2016; 91:93–104. PMID: 26689473.
Article11. Olofsson PS, Söderström LA, Wågsäter D, Sheikine Y, Ocaya P, Lang F, Rabu C, Chen L, Rudling M, Aukrust P, Hedin U, Paulsson-Berne G, Sirsjö A, Hansson GK. CD137 is expressed in human atherosclerosis and promotes development of plaque inflammation in hypercholesterolemic mice. Circulation. 2008; 117:1292–1301. PMID: 18285570.
Article12. Seo HC, Kim SM, Eo SK, Rhim BY, Kim K. 7α-hydroxycholesterol elicits TLR6-mediated expression of IL-23 in monocytic cells. Biomol Ther (Seoul). 2015; 23:84–89. PMID: 25593648.
Article13. Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000; 18:767–811. PMID: 10837075.
Article14. Palucka KA, Taquet N, Sanchez-Chapuis F, Gluckman JC. Dendritic cells as the terminal stage of monocyte differentiation. J Immunol. 1998; 160:4587–4595. PMID: 9574566.15. Chapuis F, Rosenzwajg M, Yagello M, Ekman M, Biberfeld P, Gluckman JC. Differentiation of human dendritic cells from monocytes in vitro. Eur J Immunol. 1997; 27:431–441. PMID: 9045914.16. van de Laar L, Coffer PJ, Woltman AM. Regulation of dendritic cell development by GM-CSF: molecular control and implications for immune homeostasis and therapy. Blood. 2012; 119:3383–3393. PMID: 22323450.
Article17. Bobryshev YV. Dendritic cells and their role in atherogenesis. Lab Invest. 2010; 90:970–984. PMID: 20458277.
Article18. Majmundar AJ, Skuli N, Mesquita RC, Kim MN, Yodh AG, Nguyen-McCarty M, Simon MC. O(2) regulates skeletal muscle progenitor differentiation through phosphatidylinositol 3-kinase/AKT signaling. Mol Cell Biol. 2012; 32:36–49. PMID: 22006022.19. Willimann K, Legler DF, Loetscher M, Roos RS, Delgado MB, Clark-Lewis I, Baggiolini M, Moser B. The chemokine SLC is expressed in T cell areas of lymph nodes and mucosal lymphoid tissues and attracts activated T cells via CCR7. Eur J Immunol. 1998; 28:2025–2034. PMID: 9645384.
Article20. Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci U S A. 2000; 97:12694–12699. PMID: 11070085.
Article21. Alvarez D, Vollmann EH, von Andrian UH. Mechanisms and consequences of dendritic cell migration. Immunity. 2008; 29:325–342. PMID: 18799141.
Article22. Piao M, Tokunaga O. Significant expression of endoglin (CD105), TGFbeta-1 and TGFbeta R-2 in the atherosclerotic aorta: an immunohistological study. J Atheroscler Thromb. 2006; 13:82–89. PMID: 16733295.
Article23. Lee NY, Golzio C, Gatza CE, Sharma A, Katsanis N, Blobe GC. Endoglin regulates PI3-kinase/Akt trafficking and signaling to alter endothelial capillary stability during angiogenesis. Mol Biol Cell. 2012; 23:2412–2423. PMID: 22593212.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diclofenac Inhibits 27-hydroxycholesterol-induced Differentiation of Monocytic Cells into Mature Dendritic Cells
- 7alpha-Hydroxycholesterol Elicits TLR6-Mediated Expression of IL-23 in Monocytic Cells
- Inhibitory Effects of Methanol Extract from Nardostachys chinensis on 27-hydroxycholesterol-induced Differentiation of Monocytic Cells
- FSL-1, a Toll-like Receptor 2/6 Agonist, Induces Expression of Interleukin-1alpha in the Presence of 27-hydroxycholesterol
- Differential modulation of zinc-stimulated p21(Cip/WAF1) and cyclin D1 induction by inhibition of PI3 kinase in HT-29 colorectal cancer cells