World J Mens Health.
2018 Jan;36(1):57-65. 10.5534/wjmh.17026.
Clinical Significance of Serum Adipokines according to Body Mass Index in Patients with Clinically Localized Prostate Cancer Undergoing Radical Prostatectomy
- Affiliations
-
- 1Department of Urology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Department of Urology, Seoul National University College of Medicine, Seoul, Korea. skhong@snubh.org
- 3Department of Urology, Seoul National University Bundang Hospital, Seongnam, Korea.
- KMID: 2399172
- DOI: http://doi.org/10.5534/wjmh.17026
Abstract
- PURPOSE
The aim of this study was to investigate the clinical significance of 7 circulating adipokines according to body mass index (BMI) in Korean men with localized prostate cancer (PCa) undergoing radical prostatectomy (RP).
MATERIALS AND METHODS
Sixty-two of 65 prospectively enrolled patients with clinically localized PCa who underwent RP between 2015 and 2016 were evaluated. Patients were classified into 2 groups according to their BMI: non-obese (< 25 kg/m²) and obese (≥25 kg/m²). The adipokines evaluated were interleukin-2, insulin-like growth factor 1 (IGF-1), chemerin, C-X-C motif chemokine 10, adiponectin, leptin, and resistin. Multivariate logistic regression analysis was used to identify the independent predictors of advanced tumor stage.
RESULTS
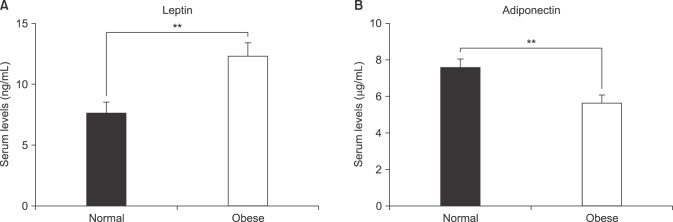
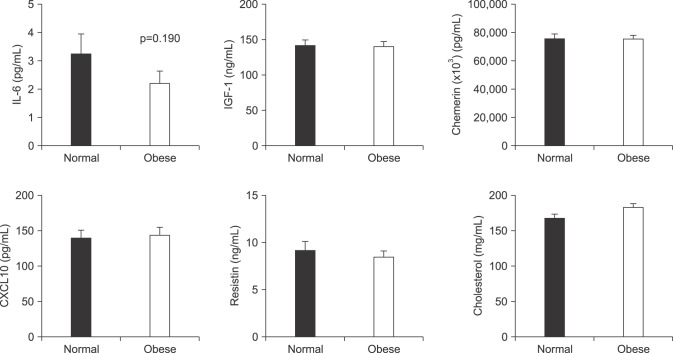
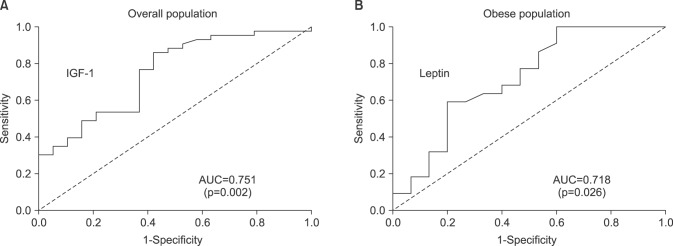
We found that obese patients with PCa who underwent RP had a higher incidence of tumors with a high Gleason score (≥8), pathological T3 (pT3) stage, and positive extraprostatic extension than patients with a normal BMI. Additionally, patients with obesity showed significantly lower serum adiponectin and higher serum leptin levels, but did not show differences in other adipokines. Multivariate analysis demonstrated that IGF-1 (odds ratio [OR]=1.03) was identified as a predictor of advanced tumor stage (≥pT3) in the overall population. However, only leptin remained an independent predictive factor for advanced tumor stage (≥pT3) (OR=1.15) in patients with obesity.
CONCLUSIONS
In conclusion, our results indicate that a higher leptin level in obese men can be considered a risk factor for aggressive PCa. This prospective study provides greater insight into the role of circulating adipokines in Korean patients with PCa undergoing RP, particularly in patients with obesity.
Keyword
MeSH Terms
-
Adipokines*
Adiponectin
Body Mass Index*
Humans
Incidence
Insulin-Like Growth Factor I
Interleukin-2
Leptin
Logistic Models
Male
Multivariate Analysis
Neoplasm Grading
Obesity
Passive Cutaneous Anaphylaxis
Prospective Studies
Prostate*
Prostatectomy*
Prostatic Neoplasms*
Resistin
Risk Factors
Adipokines
Adiponectin
Insulin-Like Growth Factor I
Interleukin-2
Leptin
Resistin
Figure
Reference
-
1. Tsujimoto T, Kajio H, Sugiyama T. Obesity, diabetes, and length of time in the United States: Analysis of National Health and Nutrition Examination Survey 1999 to 2012. Medicine (Baltimore). 2016; 95:e4578. PMID: 27583867.2. Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004; 4:579–591. PMID: 15286738.
Article3. Byers T, Sedjo RL. Body fatness as a cause of cancer: epidemiologic clues to biologic mechanisms. Endocr Relat Cancer. 2015; 22:R125–R134. PMID: 25870250.
Article4. Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol. 2013; 63:800–809. PMID: 23219374.
Article5. Parikesit D, Mochtar CA, Umbas R, Hamid AR. The impact of obesity towards prostate diseases. Prostate Int. 2016; 4:1–6. PMID: 27014656.
Article6. Golabek T, Bukowczan J, Chłosta P, Powroźnik J, Dobruch J, Borówka A. Obesity and prostate cancer incidence and mortality: a systematic review of prospective cohort studies. Urol Int. 2014; 92:7–14. PMID: 23942223.
Article7. Mistry T, Digby JE, Desai KM, Randeva HS. Obesity and prostate cancer: a role for adipokines. Eur Urol. 2007; 52:46–53. PMID: 17399889.
Article8. McGown C, Birerdinc A, Younossi ZM. Adipose tissue as an endocrine organ. Clin Liver Dis. 2014; 18:41–58. PMID: 24274864.
Article9. Baillargeon J, Rose DP. Obesity, adipokines, and prostate cancer (review). Int J Oncol. 2006; 28:737–745. PMID: 16465380.
Article10. Kim MK, Lee WY, Kang JH, Kang JH, Kim BT, Kim SM, et al. 2014 clinical practice guidelines for overweight and obesity in Korea. Endocrinol Metab (Seoul). 2014; 29:405–409. PMID: 25559568.
Article11. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008; 371:569–578. PMID: 18280327.
Article12. Discacciati A, Orsini N, Wolk A. Body mass index and incidence of localized and advanced prostate cancer: a doseresponse meta-analysis of prospective studies. Ann Oncol. 2012; 23:1665–1671. PMID: 22228452.13. Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res (Phila). 2011; 4:486–501. PMID: 21233290.
Article14. de Cobelli O, Terracciano D, Tagliabue E, Raimondi S, Galasso G, Cioffi A, et al. Body mass index was associated with upstaging and upgrading in patients with low-risk prostate cancer who met the inclusion criteria for active surveillance. Urol Oncol. 2015; 33:201.e1–201.e8.
Article15. Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med. 2010; 61:301–316. PMID: 19824817.
Article16. Alshaker H, Sacco K, Alfraidi A, Muhammad A, Winkler M, Pchejetski D. Leptin signalling, obesity and prostate cancer: molecular and clinical perspective on the old dilemma. Oncotarget. 2015; 6:35556–35563. PMID: 26376613.
Article17. Onuma M, Bub JD, Rummel TL, Iwamoto Y. Prostate cancer cell-adipocyte interaction: leptin mediates androgen-independent prostate cancer cell proliferation through c-Jun NH2-terminal kinase. J Biol Chem. 2003; 278:42660–42667. PMID: 12902351.18. Somasundar P, Frankenberry KA, Skinner H, Vedula G, McFadden DW, Riggs D, et al. Prostate cancer cell proliferation is influenced by leptin. J Surg Res. 2004; 118:71–82. PMID: 15093720.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Radical Prostatectomy
- Clinicopathological Significance of the Lymphovascular Invasion Detected in Specimens from Radical Retropubic Prostatectomies
- Clinical Significance of a Single-Core Positive Prostate Cancers Detected on Extended Prostate Needle Biopsy
- Clinical Significance of Calculated Prostate Cancer Volume as the Predictor of Pathologic Stage
- Neoadjuvant Hormonal Therapy Preceding Radical Prostatectomy for Clinically Localized Prostate Cancer: Early Postoperative Complications and Biochemical Recurrence