J Gastric Cancer.
2017 Dec;17(4):283-294. 10.5230/jgc.2017.17.e34.
Long-term Follow-up for Type 2 Diabetes Mellitus after Gastrectomy in Non-morbidly Obese Patients with Gastric Cancer: the Legitimacy of Onco-metabolic Surgery
- Affiliations
-
- 1Department of Surgery, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea. kugspss@korea.ac.kr
- KMID: 2398795
- DOI: http://doi.org/10.5230/jgc.2017.17.e34
Abstract
- PURPOSE
This study primarily aimed to investigate the short- and long-term remission rates of type 2 diabetes (T2D) in patients who underwent surgical treatment for gastric cancer, especially patients who were non-obese, and secondarily to determine the potential factors associated with remission.
MATERIALS AND METHODS
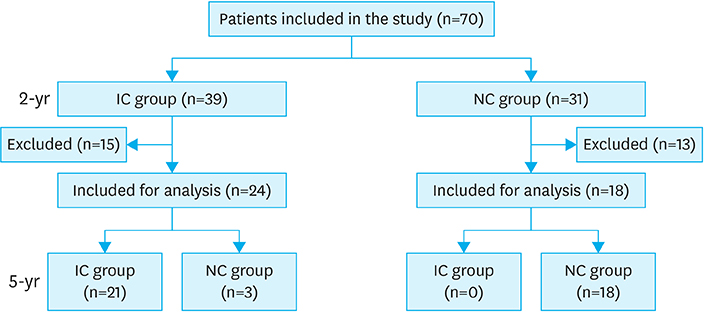
We retrospectively reviewed the clinical records of patients with T2D who underwent radical gastrectomy for gastric cancer, from January 2008 to December 2012.
RESULTS
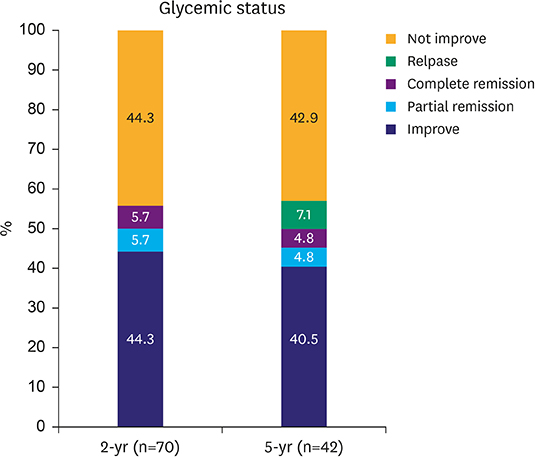
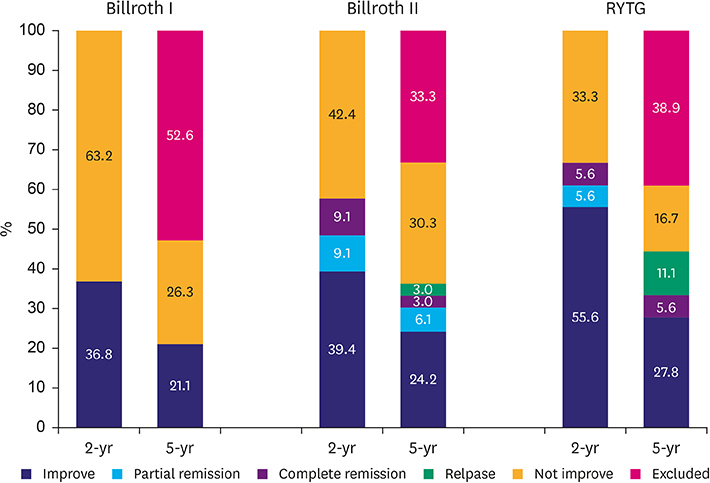
T2D improved in 39 out of 70 (55.7%) patients at the postoperative 2-year follow-up and 21 of 42 (50.0%) at the 5-year follow-up. In the 2-year data analysis, preoperative body mass index (BMI) (P=0.043), glycated hemoglobin (A1C) level (P=0.039), number of anti-diabetic medications at baseline (P=0.040), reconstruction method (statistical difference was noted between Roux-en-Y reconstruction and Billroth I; P=0.035) were significantly related to the improvement in glycemic control. Unlike the results at 2 years, the 5-year data analysis revealed that only preoperative BMI (P=0.043) and A1C level (P=0.039) were statistically significant for the improvement in glycemic control; however, the reconstruction method was not.
CONCLUSIONS
All types of gastric cancer surgery can be effective in short- and long-term T2D control in non-obese patients. In addition, unless long-limb bypass is considered in gastric cancer surgery, the long-term glycemic control is not expected to be different between the reconstruction methods.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Beneficial effects of proximal intestinal bypass reconstruction on glucose metabolism in a type 2 diabetes animal model: a possible reconstruction strategy for diabetic gastric cancer patients
Soo Min Ahn, Woo Jin Hyung
Ann Surg Treat Res. 2021;100(4):218-227. doi: 10.4174/astr.2021.100.4.218.
Reference
-
1. Choi KS, Jun JK, Suh M, Park B, Noh DK, Song SH, et al. Effect of endoscopy screening on stage at gastric cancer diagnosis: results of the National Cancer Screening Programme in Korea. Br J Cancer. 2015; 112:608–612.2. Jun JK, Choi KS, Lee HY, Suh M, Park B, Song SH, et al. Effectiveness of the Korean National Cancer Screening Program in reducing gastric cancer mortality. Gastroenterology. 2017; 152:1319–1328.e7.3. Saito T, Kurokawa Y, Takiguchi S, Mori M, Doki Y. Current status of function-preserving surgery for gastric cancer. World J Gastroenterol. 2014; 20:17297–17304.4. Tun NN, Arunagirinathan G, Munshi SK, Pappachan JM. Diabetes mellitus and stroke: a clinical update. World J Diabetes. 2017; 8:235–248.5. Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, et al. Retinopathy in diabetes. Diabetes Care. 2004; 27:Suppl 1. S84–S87.6. Ritz E, Rychlík I, Locatelli F, Halimi S. End-stage renal failure in type 2 diabetes: a medical catastrophe of worldwide dimensions. Am J Kidney Dis. 1999; 34:795–808.7. Ritz E, Stefanski A. Diabetic nephropathy in type II diabetes. Am J Kidney Dis. 1996; 27:167–194.8. Wolf G, Ritz E. Diabetic nephropathy in type 2 diabetes prevention and patient management. J Am Soc Nephrol. 2003; 14:1396–1405.9. Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004; 292:1724–1737.10. Cho JM, Kim HJ, Lo Menzo E, Park S, Szomstein S, Rosenthal RJ. Effect of sleeve gastrectomy on type 2 diabetes as an alternative treatment modality to Roux-en-Y gastric bypass: systemic review and meta-analysis. Surg Obes Relat Dis. 2015; 11:1273–1280.11. Andersen JR, Aasprang A, Karlsen TI, Natvig GK, Våge V, Kolotkin RL. Health-related quality of life after bariatric surgery: a systematic review of prospective long-term studies. Surg Obes Relat Dis. 2015; 11:466–473.12. Risstad H, Søvik TT, Engström M, Aasheim ET, Fagerland MW, Olsén MF, et al. Five-year outcomes after laparoscopic gastric bypass and laparoscopic duodenal switch in patients with body mass index of 50 to 60: a randomized clinical trial. JAMA Surg. 2015; 150:352–361.13. Baskota A, Li S, Dhakal N, Liu G, Tian H. Bariatric surgery for type 2 diabetes mellitus in patients with BMI <30 kg/m2: a systematic review and meta-analysis. PLoS One. 2015; 10:e0132335.14. Fried M, Ribaric G, Buchwald JN, Svacina S, Dolezalova K, Scopinaro N. Metabolic surgery for the treatment of type 2 diabetes in patients with BMI <35 kg/m2: an integrative review of early studies. Obes Surg. 2010; 20:776–790.15. ClinicalTrials.gov. (US). Nutritional safety and metabolic benefits of oncometabolic surgery for obese gastric cancer patients (ONCOMETAB) [Internet]. Bethesda (MD): National Institutes of Health;2017. cited 2017 Sep 26. Available from: https://clinicaltrials.gov/ct2/show/NCT03067012.16. Lee CM, Kim JH. Surgical treatment of morbid obesity. Korean J Helicobacter Up Gastrointest Res. 2017; 17:72–78.17. Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010; 17:3077–3079.18. American Diabetes Association. Standards of medical care in diabetes-2016 abridged for primary care providers. Clin Diabetes. 2016; 34:3–21.19. American Diabetes Association. Standards of medical care in diabetes-2017 abridged for primary care providers. Clin Diabetes. 2017; 35:5–26.20. Brethauer SA, Kim J, el Chaar M, Papasavas P, Eisenberg D, Rogers A, et al. Standardized outcomes reporting in metabolic and bariatric surgery. Surg Obes Relat Dis. 2015; 11:489–506.21. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011; 14:113–123.22. Lee CM, Park S, Park SH, Jang YJ, Kim SJ, Mok YJ, et al. A comparison between two methods for tumor localization during totally laparoscopic distal gastrectomy in patients with gastric cancer. Ann Surg Treat Res. 2016; 91:112–117.23. Cho YM. A gut feeling to cure diabetes: potential mechanisms of diabetes remission after bariatric surgery. Diabetes Metab J. 2014; 38:406–415.24. Pak J, Kwon Y, Lo Menzo E, Park S, Szomstein S, Rosenthal RJ. Impact of gastrointestinal bypass on nonmorbidly obese type 2 diabetes mellitus patients after gastrectomy. Surg Obes Relat Dis. 2015; 11:1266–1272.25. Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009; 122:248–256.e5.26. Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017; 3:524–548.27. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–E386.28. Aminian A, Brethauer SA, Andalib A, Punchai S, Mackey J, Rodriguez J, et al. Can sleeve gastrectomy “cure” diabetes? Long-term metabolic effects of sleeve gastrectomy in patients with type 2 diabetes. Ann Surg. 2016; 264:674–681.29. Kim JW, Kim KY, Lee SC, Yang DH, Kim BC. The effect of long Roux-en-Y gastrojejunostomy in gastric cancer patients with type 2 diabetes and body mass index < 35 kg/m(2): preliminary results. Ann Surg Treat Res. 2015; 88:215–221.30. Kwon Y, Jung Kim H, Lo Menzo E, Park S, Szomstein S, Rosenthal RJ. A systematic review and meta-analysis of the effect of Billroth reconstruction on type 2 diabetes: a new perspective on old surgical methods. Surg Obes Relat Dis. 2015; 11:1386–1395.31. Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab. 2008; 10:Suppl 4. 32–42.32. Retnakaran R, Zinman B. Short-term intensified insulin treatment in type 2 diabetes: long-term effects on β-cell function. Diabetes Obes Metab. 2012; 14:Suppl 3. 161–166.33. Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med. 2014; 370:2002–2013.34. Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, et al. Bariatric surgery versus intensive medical therapy for diabetes: 5-year outcomes. N Engl J Med. 2017; 376:641–651.35. Rao RS, Kini S. Diabetic and bariatric surgery: a review of the recent trends. Surg Endosc. 2012; 26:893–903.36. Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004; 239:1–11.37. Thomas S, Schauer P. Bariatric surgery and the gut hormone response. Nutr Clin Pract. 2010; 25:175–182.38. Kao YH, Lo CH, Huang CK. Relationship of bypassed limb length and remission of type 2 diabetes mellitus after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2012; 8:e82–e84.39. Pinheiro JS, Schiavon CA, Pereira PB, Correa JL, Noujaim P, Cohen R. Long-long limb Roux-en-Y gastric bypass is more efficacious in treatment of type 2 diabetes and lipid disorders in super-obese patients. Surg Obes Relat Dis. 2008; 4:521–525.40. Kim WS, Kim JW, Ahn CW, Choi SH. Resolution of type 2 diabetes after gastrectomy for gastric cancer with long limb Roux-en Y reconstruction: a prospective pilot study. J Korean Surg Soc. 2013; 84:88–93.41. Boza C, Muñoz R, Salinas J, Gamboa C, Klaassen J, Escalona A, et al. Safety and efficacy of Roux-en-Y gastric bypass to treat type 2 diabetes mellitus in non-severely obese patients. Obes Surg. 2011; 21:1330–1336.42. Kwon Y, Abdemur A, Lo Menzo E, Park S, Szomstein S, Rosenthal RJ. The foregut theory as a possible mechanism of action for the remission of type 2 diabetes in low body mass index patients undergoing subtotal gastrectomy for gastric cancer. Surg Obes Relat Dis. 2014; 10:235–242.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Surgical Treatment of Morbid Obesity
- Laparoscopic Gastrectomy and Transvaginal Specimen Extraction in a Morbidly Obese Patient with Gastric Cancer
- Current Available Options in Bariatric Surgery and Their Clinical Outcomes
- Current Status of Bariatric and Metabolic Surgery in Korea
- Resolution of type 2 diabetes after gastrectomy for gastric cancer with long limb Roux-en Y reconstruction: a prospective pilot study