J Vet Sci.
2017 Dec;18(4):419-429. 10.4142/jvs.2017.18.4.419.
Inhibitory effects of resveratrol on hepatitis B virus X protein-induced hepatocellular carcinoma
- Affiliations
-
- 1BK21 PLUS Program for Creative Veterinary Science Research, Research Institute for Veterinary Science and College of Veterinary Medicine, Seoul National University, Seoul 08826, Korea. chose@snu.ac.kr
- 2Institute of Laboratory Animal Resources, Seoul National University, Seoul 08826, Korea.
- 3College of Pharmacy, Seoul National University, Seoul 08826, Korea.
- 4Advanced Institutes of Convergence Technology, Seoul National University, Seoul 08826, Korea.
- KMID: 2398500
- DOI: http://doi.org/10.4142/jvs.2017.18.4.419
Abstract
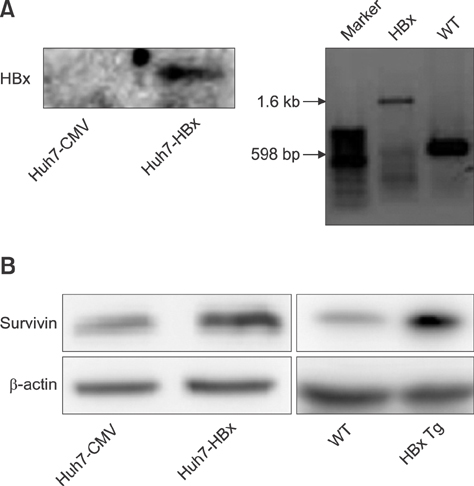
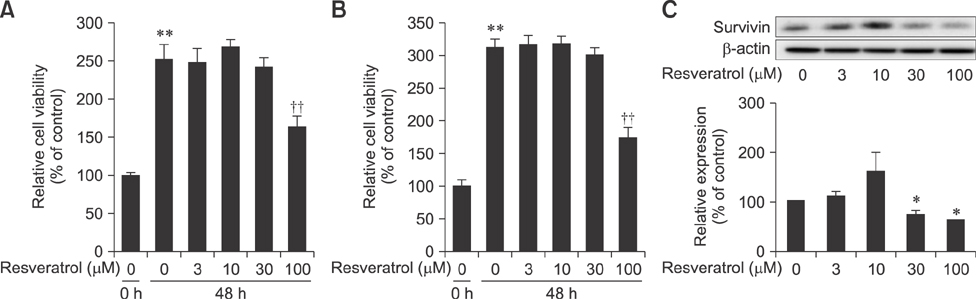
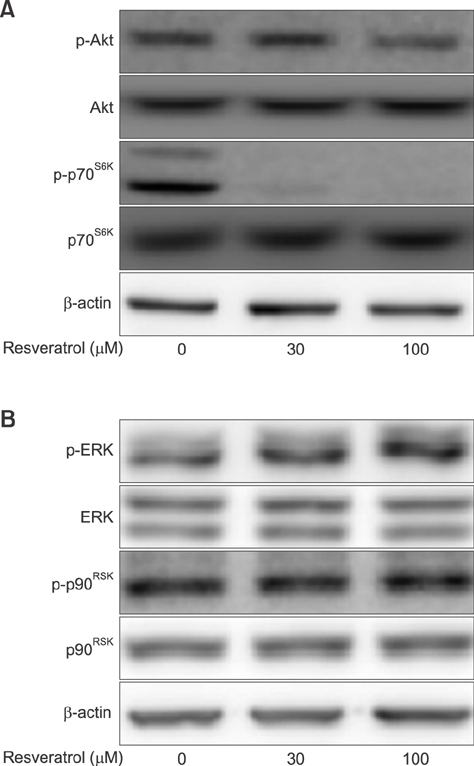
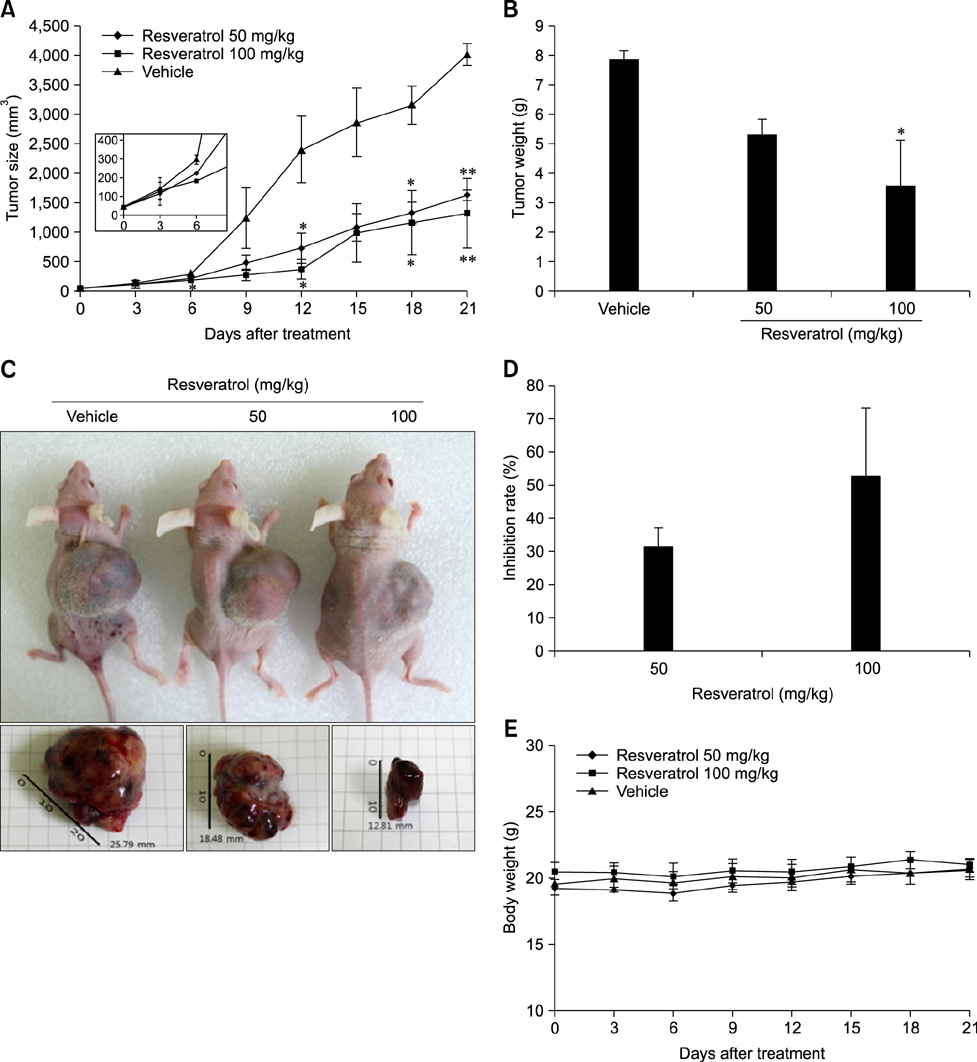
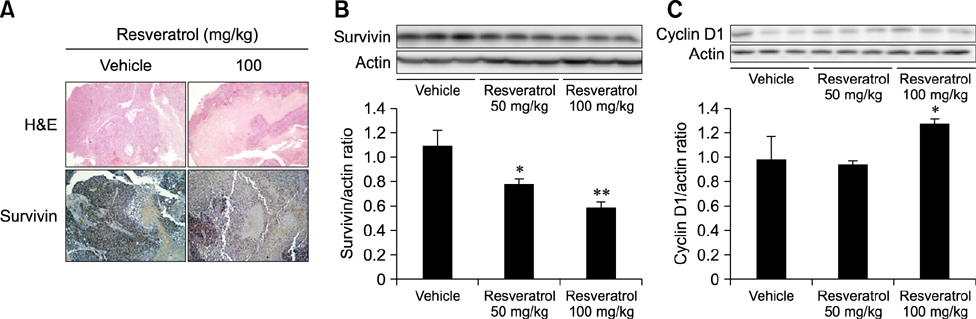
- Liver cancer occurs very frequently worldwide and hepatocellular carcinoma (HCC) accounts for more than 80% of total primary liver cancer cases. In this study, the anticarcinogenic effects of resveratrol against hepatitis B virus (HBV)-induced HCC were investigated by using HBV X-protein-overexpressing Huh7 (Huh7-HBx) human hepatoma cells. MTT assay showed that resveratrol decreased cell viability. Fluorescence-activated cell-sorter analysis showed that resveratrol induced G1 cell cycle arrest without increasing the sub-G1 phase cell population. Therefore, we evaluated its effect on regulation of cyclin D1, which is critically involved in G1/S transition. Resveratrol lowered cyclin D1 transcription. Western blot analysis of the effects of resveratrol on upstream cyclin D1 transcriptional signaling, extracellular signal-related kinase (ERK), p90(RSK), Akt, and p70(S6K) revealed inhibition of Akt but not the ERK signaling pathway. Collectively, the results indicate that resveratrol inhibits Huh7-HBx proliferation by decreasing cyclin D1 expression through blockade of Akt signaling. We investigated the anticarcinogenic effect and mechanism of resveratrol in xenograft model mice implanted with Huh7-HBx cells. Intraperitoneal resveratrol injection reduced tumor size in the mice. Expression of survivin was reduced, but cyclin D1 was not affected. The results demonstrate that resveratrol treatment may help manage HBV-induced HCC by regulating survivin.
MeSH Terms
-
Animals
Anticarcinogenic Agents
Blotting, Western
Carcinoma, Hepatocellular*
Cell Survival
Cyclin D1
G1 Phase Cell Cycle Checkpoints
Hepatitis B virus*
Hepatitis B*
Hepatitis*
Heterografts
Humans
Liver Neoplasms
Mice
Phosphotransferases
Ribosomal Protein S6 Kinases, 90-kDa
Anticarcinogenic Agents
Cyclin D1
Phosphotransferases
Ribosomal Protein S6 Kinases, 90-kDa
Figure
Reference
-
1. Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008; 8:61–70.
Article2. Altieri DC. The case for survivin as a regulator of microtubule dynamics and cell-death decisions. Curr Opin Cell Biol. 2006; 18:609–615.
Article3. Bashir T, Pagano M. Aberrant ubiquitin-mediated proteolysis of cell cycle regulatory proteins and oncogenesis. Adv Cancer Res. 2003; 88:101–144.
Article4. Blumberg BS, Larouzé B, London WT, Werner B, Hesser JE, Millman I, Saimot G, Payet M. The relation of infection with the hepatitis B agent to primary hepatic carcinoma. Am J Pathol. 1975; 81:669–682.
Article5. Bobrowska-Hägerstrand M, Lillås M, Mrówczyñska L, Wróbel A, Shirataki Y, Motohashi N, Hägerstrand H. Resveratrol oligomers are potent MRP1 transport inhibitors. Anticancer Res. 2006; 26:2081–2084.6. Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005; 42:1208–1236.
Article7. Chirillo P, Pagano S, Natoli G, Puri PL, Burgio VL, Balsano C, Levrero M. The hepatitis B virus X gene induces p53-mediated programmed cell death. Proc Natl Acad Sci U S A. 1997; 94:8162–8167.8. Choi KH, Kim JE, Song NR, Son JE, Hwang MK, Byun S, Kim JH, Lee KW, Lee HJ. Phosphoinositide 3-kinase is a novel target of piceatannol for inhibiting PDGF-BB-induced proliferation and migration in human aortic smooth muscle cells. Cardiovasc Res. 2010; 85:836–844.
Article9. Dai Z, Lei P, Xie J, Hu Y. Antitumor effect of resveratrol on chondrosarcoma cells via phosphoinositide 3-kinase/AKT and p38 mitogen-activated protein kinase pathways. Mol Med Rep. 2015; 12:3151–3155.
Article10. Eckelman BP, Salvesen GS, Scott FL. Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep. 2006; 7:988–994.
Article11. Espinoza JL, Takami A, Trung LQ, Kato S, Nakao S. Resveratrol prevents EBV transformation and inhibits the outgrowth of EBV-immortalized human B cells. PLoS One. 2012; 7:e51306.
Article12. Guerrieri F, Belloni L, Pediconi N, Levrero M. Molecular mechanisms of HBV-associated hepatocarcinogenesis. Semin Liver Dis. 2013; 33:147–156.
Article13. Huh WB, Kim JE, Kang YG, Park G, Lim TG, Kwon JY, Song da S, Jeong EH, Lee CC, Son JE, Seo SG, Lee E, Kim JR, Lee CY, Park JS, Lee KW. Brown pine leaf extract and its active component trans-communic acid inhibit UVB-induced MMP-1 expression by targeting PI3K. PLoS One. 2015; 10:e0128365.14. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
Article15. Kew MC. Epidemiology of chronic hepatitis B virus infection, hepatocellular carcinoma, and hepatitis B virus-induced hepatocellular carcinoma. Pathol Biol (Paris). 2010; 58:273–277.
Article16. Knoll S, Fürst K, Thomas S, Villanueva Baselga S, Stoll A, Schaefer S, Pützer BM. Dissection of cell context-dependent interactions between HBx and p53 family members in regulation of apoptosis: a role for HBV-induced HCC. Cell Cycle. 2011; 10:3554–3565.
Article17. Kolodziej M, Goetz C, Di Fazio P, Montalbano R, Ocker M, Strik H, Quint K. Roscovitine has anti-proliferative and pro-apoptotic effects on glioblastoma cell lines: a pilot study. Oncol Rep. 2015; 34:1549–1556.
Article18. Lee DE, Lee KW, Jung SK, Lee EJ, Hwang JA, Lim TG, Kim BY, Bode AM, Lee HJ, Dong Z. 6,7,4′-trihydroxyisoflavone inhibits HCT-116 human colon cancer cell proliferation by targeting CDK1 and CDK2. Carcinogenesis. 2011; 32:629–635.
Article19. Lee H, Zhang P, Herrmann A, Yang C, Xin H, Wang Z, Hoon DS, Forman SJ, Jove R, Riggs AD, Yu H. Acetylated STAT3 is crucial for methylation of tumor-suppressor gene promoters and inhibition by resveratrol results in demethylation. Proc Natl Acad Sci U S A. 2012; 109:7765–7769.
Article20. Lin HC, Chen YF, Hsu WH, Yang CW, Kao CH, Tsai TF. Resveratrol helps recovery from fatty liver and protects against hepatocellular carcinoma induced by hepatitis B virus X protein in a mouse model. Cancer Prev Res (Phila). 2012; 5:952–962.
Article21. O'Dell MR, Huang JL, Whitney-Miller CL, Deshpande V, Rothberg P, Grose V, Rossi RM, Zhu AX, Land H, Bardeesy N, Hezel AF. KrasG12D and p53 mutation cause primary intrahepatic cholangiocarcinoma. Cancer Res. 2012; 72:1557–1567.22. Parekh P, Motiwale L, Naik N, Rao KV. Downregulation of cyclin D1 is associated with decreased levels of p38 MAP kinases, Akt/PKB and Pak1 during chemopreventive effects of resveratrol in liver cancer cells. Exp Toxicol Pathol. 2011; 63:167–173.
Article23. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005; 55:74–108.
Article24. Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006; 45:529–538.
Article25. Quoc Trung L, Espinoza JL, Takami A, Nakao S. Resveratrol induces cell cycle arrest and apoptosis in malignant NK cells via JAK2/STAT3 pathway inhibition. PLoS One. 2013; 8:e55183.
Article26. Seo SG, Yang H, Shin SH, Min S, Kim YA, Yu JG, Lee DE, Chung MY, Heo YS, Kwon JY, Yue S, Kim KH, Cheng JX, Lee KW, Lee HJ. A metabolite of daidzein, 6,7,4′-trihydroxyisoflavone, suppresses adipogenesis in 3T3-L1 preadipocytes via ATP-competitive inhibition of PI3K. Mol Nutr Food Res. 2013; 57:1446–1455.
Article27. Shapiro GI. Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol. 2006; 24:1770–1783.
Article28. Su F, Schneider RJ. Hepatitis B virus HBx protein sensitizes cells to apoptotic killing by tumor necrosis factor α. Proc Natl Acad Sci U S A. 1997; 94:8744–8749.
Article29. Sun W, Zhong F, Zhi L, Zhou G, He F. Systematic-omics analysis of HBV-associated liver diseases. Cancer Lett. 2009; 286:89–95.30. Tian Y, Yang W, Song J, Wu Y, Ni B. Hepatitis B virus X protein-induced aberrant epigenetic modifications contributing to human hepatocellular carcinoma pathogenesis. Mol Cell Biol. 2013; 33:2810–2816.
Article31. Tsutsui M, Yasuda H, Suto H, Imai H, Isobe Y, Sasaki M, Kojima Y, Oshimi K, Sugimoto K. Frequent STAT3 activation is associated with Mcl-1 expression in nasal NK-cell lymphoma. Int J Lab Hematol. 2010; 32:419–426.
Article32. Vastano BC, Chen Y, Zhu N, Ho CT, Zhou Z, Rosen RT. Isolation and identification of stilbenes in two varieties of Polygonum cuspidatum. J Agric Food Chem. 2000; 48:253–256.
Article33. Wicklow B, Wittmeier K, t' Jong GW, McGavock J, Robert M, Duhamel T, Dolinsky VW. Proposed trial: safety and efficacy of resveratrol for the treatment of non-alcoholic fatty liver disease (NAFLD) and associated insulin resistance in adolescents who are overweight or obese adolescents — rationale and protocol. Biochem Cell Biol. 2015; 93:522–530.
Article34. Wu CF, Yu MW, Lin CL, Liu CJ, Shih WL, Tsai KS, Chen CJ. Long-term tracking of hepatitis B viral load and the relationship with risk for hepatocellular carcinoma in men. Carcinogenesis. 2008; 29:106–112.
Article35. Yang HI, Lu SN, Liaw YF, You SL, Sun CA, Wang LY, Hsiao CK, Chen PJ, Chen DS, Chen CJ. Taiwan Community-Based Cancer Screening Project Group. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002; 347:168–174.
Article36. Yu DY, Moon HB, Son JK, Jeong S, Yu SL, Yoon H, Han YM, Lee CS, Park JS, Lee CH, Hyun BH, Murakami S, Lee KK. Incidence of hepatocellular carcinoma in transgenic mice expressing the hepatitis B virus X-protein. J Hepatol. 1999; 31:123–132.
Article37. Yu HB, Zhang HF, Zhang X, Li DY, Xue HZ, Pan CE, Zhao SH. Resveratrol inhibits VEGF expression of human hepatocellular carcinoma cells through a NF-kappa B-mediated mechanism. Hepatogastroenterology. 2010; 57:1241–1246.38. Zhang W, Cai N, Ye L, Zhang X. Transformation of human liver L-O2 cells mediated by stable HBx transfection. Acta Pharmacol Sin. 2009; 30:1153–1161.
Article39. Zhang W, Lu Z, Kong G, Gao Y, Wang T, Wang Q, Cai N, Wang H, Liu F, Ye L, Zhang X. Hepatitis B virus X protein accelerates hepatocarcinogenesis with partner survivin through modulating miR-520b and HBXIP. Mol Cancer. 2014; 13:128.
Article40. Zhang X, Dong N, Yin L, Cai N, Ma H, You J, Zhang H, Wang H, He R, Ye L. Hepatitis B virus X protein upregulates survivin expression in hepatoma tissues. J Med Virol. 2005; 77:374–381.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of primary hepatocellular carcinoma following vertical transmission of hepatitis B virus in a child
- Serum Hepatitis B Virus DHA Level and Hepatocellulor Carcinoma
- Relationships among Hepatitis C Virus, Hepatocellular Carcinoma, and Diffuse Large B Cell Lymphoma: A Case Report
- The use of transient elastography for predicting hepatocellular carcinoma in chronic hepatitis B patients: Editorial on “Risk assessment of hepatitis B virus-related hepatocellular carcinoma development using vibration-controlled transient elastography: Systematic review and meta-analysis”
- Cyclophilin A as a New Therapeutic Target for Hepatitis C Virus-induced Hepatocellular Carcinoma