Asia Pac Allergy.
2011 Apr;1(1):16-24. 10.5415/apallergy.2011.1.1.16.
Clinical characteristics and long-term outcomes related to sputum eosinophilia in Korean asthmatics
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul 110-799, Korea. shcho@plaza.snu.ac.kr
- 2Institute of Allergy and Clinical Immunology, Seoul National University Medical Research Center, Seoul 110-799, Korea.
- 3Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam 463-707, Korea.
- KMID: 2397141
- DOI: http://doi.org/10.5415/apallergy.2011.1.1.16
Abstract
- BACKGROUND
Bronchial asthma is usually associated with high sputum eosinophil levels. However, recent reports have suggested the importance of noneosinophilic asthma (NEA) as a distinct phenotype of asthma.
OBJECTIVE
The aim of this study was to evaluate clinical significance of sputum eosinophilia and long-term treatment outcomes related to sputum eosinophilia in Korean asthmatics.
METHODS
A total of 201 steroid-naive asthmatics who had undergone induced sputum analysis at baseline were selected from the Cohort for Reality and Evolution of Adult Asthma study population. Clinical evaluation, spirometry, a skin-prick test, a methacholine bronchial provocation test, and sputum eosinophil analysis were performed initially, and patients received the treatment recommended by the Global Initiative for Asthma. Lung function was evaluated every 6 months, and 53 patients completed 24 months of regular follow-up visits. Sputum eosinophilia was defined as a sputum eosinophil count of >3%.
RESULTS
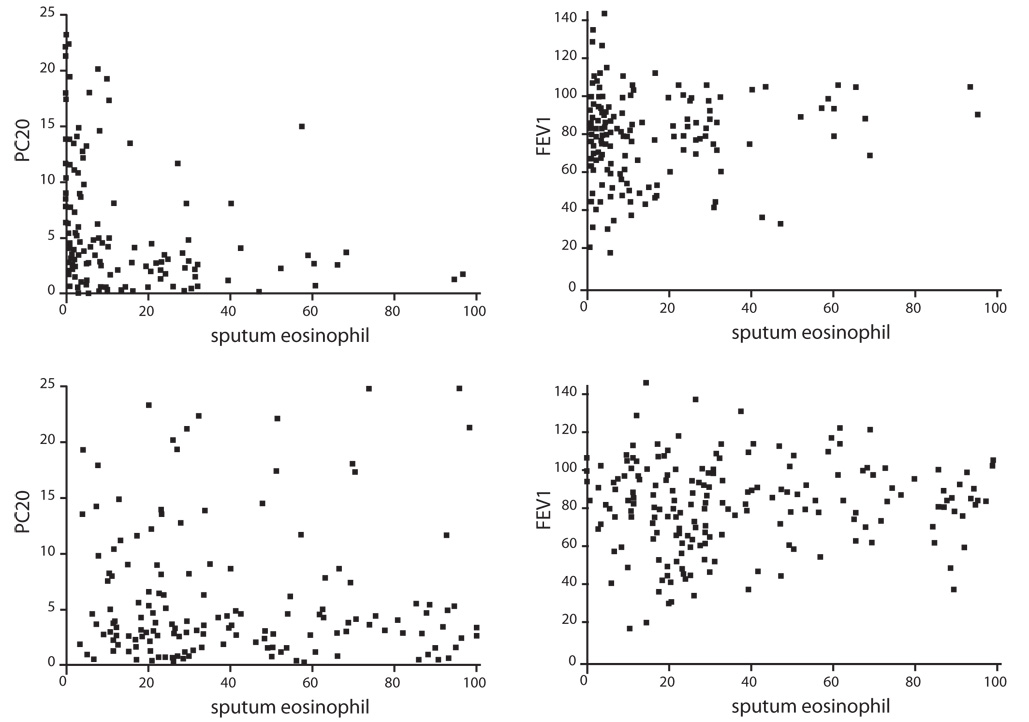
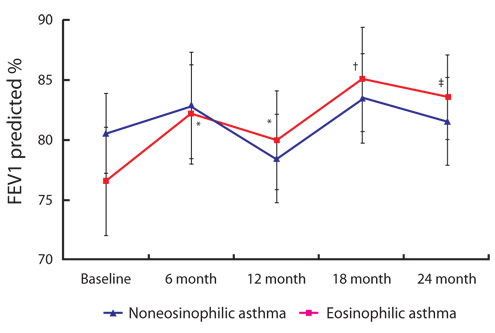
Of the 201 steroid-naive asthmatics, 97 patients had NEA and 104 had eosinophilic asthma (EA). Only 52% of steroid-naive asthmatic subjects had elevated baseline sputum eosinophil levels. A higher percentage of sputum eosinophils was associated with a lower PC20 (r = -0.193; p = 0.009, Spearman correlation), but not with forced expiratory volume in one second (FEV1) (r = 0.045; p = 0.525). During the 24-month study period, the percentage change of FEV1 was significantly lower in the NEA group than in the EA group at 6, 12, 18, and 24 months (p < 0.05). The NEA group, unlike the EA group, showed no significant improvement in FEV1 at 6, 12, 18, or 24 months (p > 0.05).
CONCLUSION
A higher sputum eosinophil percentage was correlated with a higher airway hyperresponsiveness. Compared with EA patients, NEA patients had poor treatment outcomes in the 2-year follow-up of a Korean asthma cohort population.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Association Between Clinical Burden and Blood Eosinophil Counts in Asthma: Findings From a Korean Adult Asthma Cohort
Mi-Yeong Kim, Eun-Jung Jo, Sujeong Kim, Min-Hye Kim, Jae-Woo Jung, Joo-Hee Kim, Ji-Yong Moon, Jae-Woo Kwon, Jae-Hyun Lee, Chan Sun Park, Hyun Jung Jin, Yoo Seob Shin, Sae-Hoon Kim, Young-Joo Cho, Jung-Won Park, Sang-Heon Cho, Tae-Bum Kim, Hye-Kyung Park
J Korean Med Sci. 2022;37(7):e57. doi: 10.3346/jkms.2022.37.e57.
Reference
-
1. Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006; 368:804–813.
Article2. Green RH, Brightling CE, Bradding P. The reclassification of asthma based on subphenotypes. Curr Opin Allergy Clin Immunol. 2007; 7:43–50.
Article3. Fahy JV. Eosinophilic and neutrophilic inflammation in asthma: insights from clinical studies. Proc Am Thorac Soc. 2009; 6:256–259.
Article4. Holt PG, Macaubas C, Stumbles PA, Sly PD. The role of allergy in the development of asthma. Nature. 1999; 402:B12–B17.
Article5. Djukanović R, Roche WR, Wilson JW, Beasley CR, Twentyman OP, Howarth RH, Holgate ST. Mucosal inflammation in asthma. Am Rev Respir Dis. 1990; 142:434–457.
Article6. Bentley AM, Hamid Q, Robinson DS, Schotman E, Meng Q, Assoufi B, Kay AB, Durham SR. Prednisolone treatment in asthma. Reduction in the numbers of eosinophils, T cells, tryptase-only positive mast cells, and modulation of IL-4, IL-5, and interferon-gamma cytokine gene expression within the bronchial mucosa. Am J Respir Crit Care Med. 1996; 153:551–556.
Article7. Beasley R, Roche WR, Roberts JA, Holgate ST. Cellular events in the bronchi in mild asthma and after bronchial provocation. Am Rev Respir Dis. 1989; 139:806–817.
Article8. Bousquet J, Chanez P, Lacoste JY, Barnéon G, Ghavanian N, Enander I, Venge P, Ahlstedt S, Simony-Lafontaine J, Godard P, Michel FB. Eosinophilic inflammation in asthma. N Engl J Med. 1990; 323:1033–1039.
Article9. Grootendorst DC, Sont JK, Willems LN, Kluin-Nelemans JC, Van Krieken JH, Veselic-Charvat M, Sterk PJ. Comparison of inflammatory cell counts in asthma: induced sputum vs bronchoalveolar lavage and bronchial biopsies. Clin Exp Allergy. 1997; 27:769–779.
Article10. Keatings VM, Evans DJ, O'Connor BJ, Barnes PJ. Cellular profiles in asthmatic airways: a comparison of induced sputum, bronchial washings, and bronchoalveolar lavage fluid. Thorax. 1997; 52:372–374.
Article11. Green RH, Brightling CE, Woltmann G, Parker D, Wardlaw AJ, Pavord ID. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax. 2002; 57:875–879.
Article12. Turner MO, Hussack P, Sears MR, Dolovich J, Hargreave FE. Exacerbations of asthma without sputum eosinophilia. Thorax. 1995; 50:1057–1061.
Article13. Wenzel SE, Schwartz LB, Langmack EL, Halliday JL, Trudeau JB, Gibbs RL, Chu HW. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. 1999; 160:1001–1008.
Article14. Gibson PG, Simpson JL, Saltos N. Heterogeneity of airway inflammation in persistent asthma: evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest. 2001; 119:1329–1336.15. Douwes J, Gibson P, Pekkanen J, Pearce N. Non-eosinophilic asthma: importance and possible mechanisms. Thorax. 2002; 57:643–648.
Article16. Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, Wardlaw AJ, Pavord ID. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002; 360:1715–1721.
Article17. Haldar P, Pavord ID. Noneosinophilic asthma: a distinct clinical and pathologic phenotype. J Allergy Clin Immunol. 2007; 119:1043–1052.
Article18. Kim TB, Park CS, Bae YJ, Cho YS, Moon HB. Factors associated with severity and exacerbation of asthma: a baseline analysis of the cohort for reality and evolution of adult asthma in Korea (COREA). Ann Allergy Asthma Immunol. 2009; 103:311–317.
Article19. Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O'Byrne P, Pedersen SE, Pizzichini E, Sullivan SD, Wenzel SE, Zar HJ. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008; 31:143–178.
Article20. Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004; 23:932–946.
Article21. Sohn SW, Lee HS, Park HW, Chang YS, Kim YK, Cho SH, Kim YY, Min KU. Evaluation of cytokine mRNA in induced sputum from patients with allergic rhinitis: relationship to airway hyperresponsiveness. Allergy. 2008; 63:268–273.22. Simpson JL, McElduff P, Gibson PG. Assessment and reproducibility of non-eosinophilic asthma using induced sputum. Respiration. 2010; 79:147–151.
Article23. Louis R, Lau LC, Bron AO, Roldaan AC, Radermecker M, Djukanović R. The relationship between airways inflammation and asthma severity. Am J Respir Crit Care Med. 2000; 161:9–16.
Article24. Woodruff PG, Khashayar R, Lazarus SC, Janson S, Avila P, Boushey HA, Segal M, Fahy JV. Relationship between airway inflammation, hyperresponsiveness, and obstruction in asthma. J Allergy Clin Immunol. 2001; 108:753–758.
Article25. Berry M, Morgan A, Shaw DE, Parker D, Green R, Brightling C, Bradding P, Wardlaw AJ, Pavord ID. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax. 2007; 62:1043–1049.
Article26. Shaw DE, Berry MA, Hargadon B, McKenna S, Shelley MJ, Green RH, Brightling CE, Wardlaw AJ, Pavord ID. Association between neutrophilic airway inflammation and airflow limitation in adults with asthma. Chest. 2007; 132:1871–1875.
Article27. Bacci E, Cianchetti S, Bartoli M, Dente FL, Di Franco A, Vagaggini B, Paggiaro P. Low sputum eosinophils predict the lack of response to beclomethasone in symptomatic asthmatic patients. Chest. 2006; 129:565–572.
Article28. Little SA, Chalmers GW, MacLeod KJ, McSharry C, Thomson NC. Non-invasive markers of airway inflammation as predictors of oral steroid responsiveness in asthma. Thorax. 2000; 55:232–234.
Article29. Meijer RJ, Postma DS, Kauffman HF, Arends LR, Koëter GH, Kerstjens HA. Accuracy of eosinophils and eosinophil cationic protein to predict steroid improvement in asthma. Clin Exp Allergy. 2002; 32:1096–1103.
Article30. Pavord ID. Non-eosinophilic asthma and the innate immune response. Thorax. 2007; 62:193–194.
Article31. McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, Henry A, Irvin CG, Piganelli JD, Ray A, Kolls JK. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008; 181:4089–4097.
Article32. Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, Ceuppens JL. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006; 7:135.
Article33. Alcorn JF, Crowe CR, Kolls JK. TH17 cells in asthma and COPD. Annu Rev Physiol. 2010; 72:495–516.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Measurement of house dust mite-specific IgA antibody in sputum from asthmatics
- Significance of sputum eosinophils in bronchial asthmatics
- Change of Inflammatory Markers in Induced Sputum in Suspected Asthmatics by Asthma Treatment
- Sputum induction method for studying total IgE levels in atopics asthamtic patients
- Relationship between Dendritic Cells and Activated Eosinophils in Induced Sputum of Asthmatics