Nat Prod Sci.
2017 Sep;23(3):151-156. 10.20307/nps.2017.23.3.151.
New Azafluorenone Derivative and Antibacterial Activities of Alphonsea cylindrica Barks
- Affiliations
-
- 1Department of Chemistry, Faculty of Science and Mathematics, Sultan Idris Education University, 35900 Tanjung Malim, Perak Darul Ridzuan, Malaysia. saripah@fsmt.upsi.edu.my
- 2Department of Biology, Faculty of Science and Mathematics, Sultan Idris Education University, 35900 Tanjung Malim, Perak Darul Ridzuan, Malaysia.
- 3Department of Chemistry, Faculty of Science, University of Malaya, 50603 Kuala Lumpur, Malaysia.
- 4Atta-ur-Rahman Institute of Natural Product Discovery and Faculty of Pharmacy, University Technology MARA, Puncak Alam, 42300 Selangor, Malaysia.
- 5Centre de Recherche de Gif, Institute de Chimie des Substances Naturelles, CNRS, 1, Avenue de la Terrasse, 91198 Gif-sur-Yvette Cedex, France.
- KMID: 2393794
- DOI: http://doi.org/10.20307/nps.2017.23.3.151
Abstract
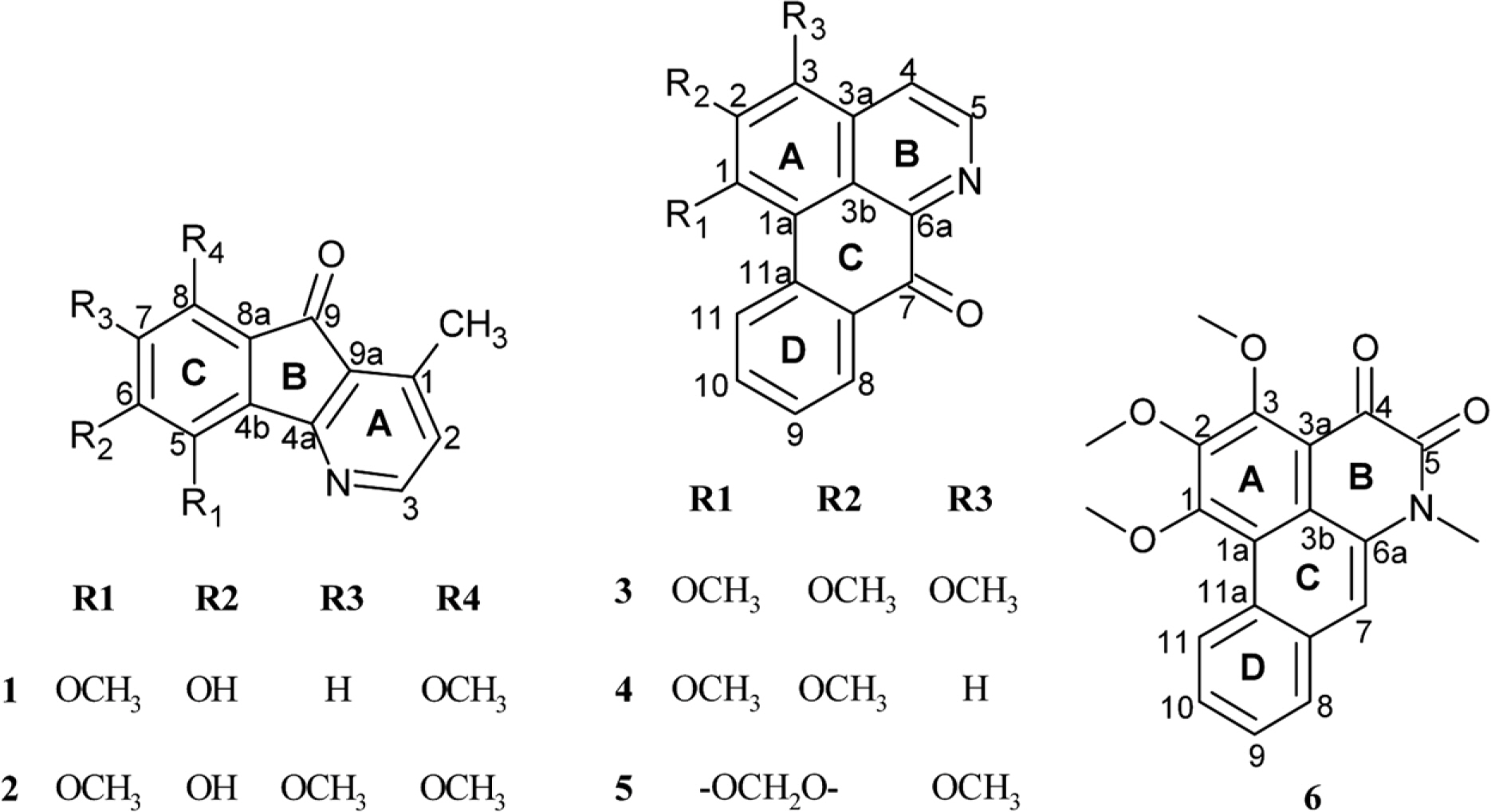
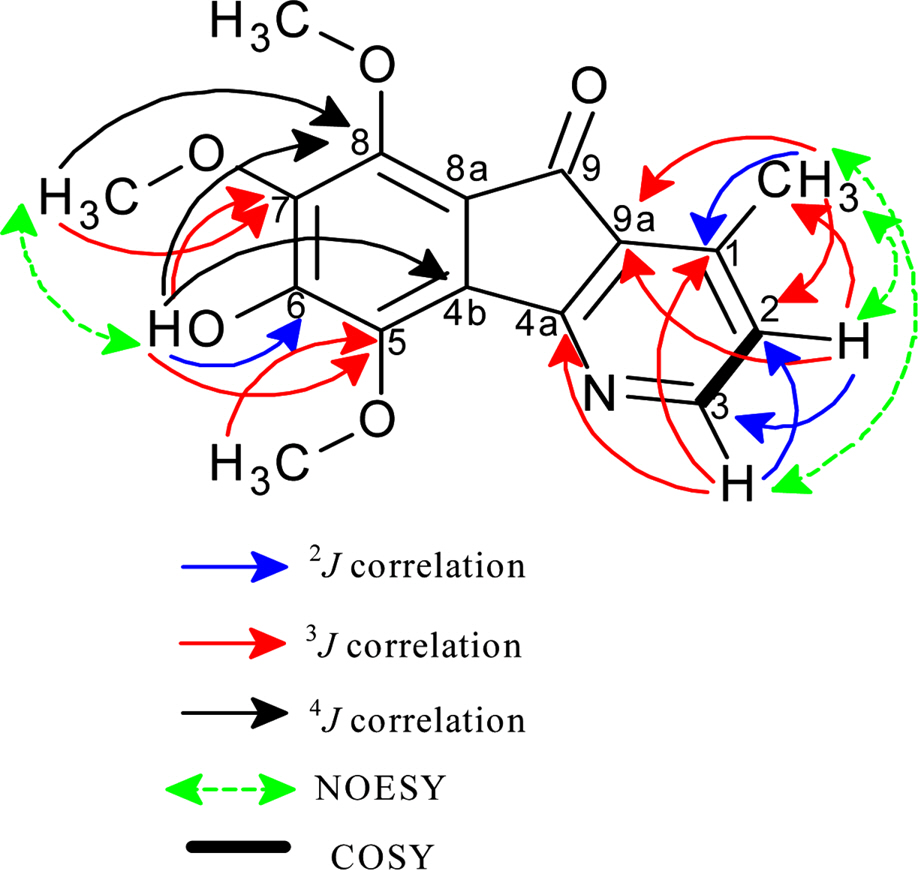
- A phytochemical study of Alphonsea cylindrica King (unreported) has led to the isolation of six alkaloids. The compounds were identified as kinabaline (1; azafluorenone alkaloid), muniranine (2), O-methylmoschatoline (3; oxoaporphine alkaloid), lysicamine (4), atherospermidine (5) and N-methylouregidione (6; 4, 5-dioxoaporphine alkaloid). The structures of the isolated compounds were determined based on the spectroscopic techniques and by comparison with data reported in the literature. Alkaloid 2 was isolated as a new derivative of azafluorenone while alkaloids 1, 3 - 6 were isolated for the first time from Alphonsea species. In addition, alkaloid 3 and 4 showed inhibition zone against Staphylococcus aureus, Pseudomonas aeruginosa and Bacillus cereus in disc diffusion test. The minimum inhibition concentration (MIC) values of lysicamine (4) against S. aureus, B. cereus and P. aeruginosa were found to be smaller than O-methylmoschatoline (3). Therefore, the reported antibacterial activity showed the potential of this plant as natural antibacterial agent and supported the documented traditional use of Alphonsea sp. in the treatment of diarrhea and fever.
Keyword
MeSH Terms
Figure
Reference
-
References
(1). Srivastava G.., Mehrotra R. C.PLoS ONE. 2013. 8:1–6.(2). Turner I. M.Gard. Bull. Singapore. 2009. 61:185–188.(3). Latiff A.Malay. Nat. J. 2013. 65:247–273.(4). Turner, I. M; Utteridge T. M. A.Blumea. 2015. 59:206–208.(5). Turner I. M.Gard. Bull. Singapore. 2016. 68:65–69.(6). IUCN Red List of Threatened Species. 2010.(7). Batugal P. A.., Jayashree K.., Lee S. Y.., Jeffrey T. O.Medicinal Plants Research in Asia, Volume 1: The Framework and Project Work plans. International Plant Genetic Resources Institute –Regional Office for Asia; the Pacific and Oceania (IPGRI-APO), Malaysia,. 2004. 3–6.(8). Jalil J.., Teh C. H.., Hussain K.., Jamal J. A.., Mohamad H. F.., Muhammad K.Abstract of International Conference on Natural Products. 2015. 154.(9). Thang T. D.., Huong L. T.., Dai D. N.., Oguwande I. A.Nat. Prod. Res. 2013. 27:2022–2026.(10). Johnson T. A.., Sohn J.., Ward A. E.., Cohen T. L.., Lorig-Roach N. D.., Chen H.., Pilli R. A.., Widjaja E. A.., Hanafi M.., Kardono L. B. S.., Lotulung P. D.., Boundy-Mills K.., Bjeldanes L. F.Bioorg. Med. Chem. 2013. 21:4358–4364.(11). Xie N.., Xu R.., Zhong S.., Zhao S.Journal-china Pharmaceutical University,. 1994. 25:205.(12). Bently K. W.Nat. Prod. Rep. 2001. 18:148–170.
Article(13). Xie N.., Zhong S.., Zhao S.., Peter G. W. J.China Pharm. Uni. 1989. 20:321–324.(14). Yang N.., Xie N.., Zhi F. J.China Pharm. Uni. 1999. 30:171–173.(15). Yang N. Y.., Xie N.., Kong L. Y.., Li G.Chin. Chem. Lett. 2000. 11:409.(16). Ning X.., Yang N. Y.Chin. Chem. Lett. 1999. 10:671–672.(17). Mahanta P. K.., Mathur R. K.., Gopinath K. W.Indian J. Chem. 1975. 13:306–308.(18). Tadi D.., Wanningama G. P.., Cassels B. K.., Cavé A. J.Nat. cí Prod. 1987. 50:518–519.(19). Gopinath K. W.., Mahanta P. K.., Bohlmann F.., Zedro C.Tetrahedron. 1976. 32:737–740.(20). Narendra P. D.Res. J. Pharmacol. Pharmacodyn. 2009. 1:66–69.(21). Horgen F. D.., Edrada R. A.., de los Reyes G.., Agcaoili F.., Madulid D. A.., Wongpanich V.., Angerhofer C. K.., Pezzuto J. M.., Soejarto D. D.., Farnsworth N. R.Phytomedicine. 2001. 8:71–81.(22). Indrani V.., Madhuri T.., Lakshmi K. B.., Suvarnalatha D. P.Int. J. Sci. Res. Management. 2015. 3:2103–2105.(23). Norhayati I.., Getha K.., Haffiz J. M.., Ilham A. M.., Sahira H. L.., Siti Syarifah M. M.., Syamil M. A. J.Trop. For. Sci. 2013. 25:52–59.(24). Hanum F. I.., Ibrahim A. Z.., Khamis S.., Nazre M.., Lepun P.., Rusea G.., Lajuni J. J.., Latiff A. Pertanika J.Trop. Agric. Sci. 2001. 24:63–78.(25). Sati S. C.., Khulbe K.., Joshi S.Res. J. Microbiol. 2011. 6:289–296.(26). European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Clin. Microbiol. Infect. 2000. 9:1–7.(27). Yusof H.., Din L. B.., Yaacob W. A.., Ibrahim N.., Yamin B. M.., Latiff A.Sains Malaysiana. 2015. 44:1125–1128.(28). Husain K.., Jamal J. A.., Jalil J.Int. J. Pharm. Pharm. Sci. 2012. 4:465–467.(29). Costa E. V.., Marques F. D. A.., Pinheiro M. L. B.., Braga R. M.., Delarmelina C.., Duarte M. C. T.., Ruiz A. L. T. G.., V; de Carvalho J. E.., Maia B. H. J.Braz. Chem. Soc. 2011. 22:1111–1117.(30). Wirasathien L.., Boonarkart C.., Pengsuparp T.., Suttisri R.Pharm. Biol. 2006. 44:274–278.(31). Tadi D.., Cassels B. K.., Leboeuf M.., Cavé A.Phytochemistry cí. 1987. 26:537–541.(32). Yoshida N. C.., de Siqueira J. M.., Rodrigues R. P.., Correia R. P.., Garcez W. S. J.Braz. Chem. Soc. 2013. 24:529–533.(33). Tadi D.., Cassels B. K.., Cavé A.Heterocycles. 1988. 27:407–c. í 421.(34). Tan K. K.., Khoo T. J.., Rajagopal M.., Wiart C.Nat. Prod. Res. 2015. 29:2346–2349.(35). Omar H.., Hashim N. M.., Zajmi A.., Nordin N.., Abdelwahab S. I.., Azizan A. H.., Hadi, A. H; Ali H. M.Molecules. 2013. 18:8994–9009.(36). Tavares Lde C.., Zanon G.., Weber A. D.., Neto A. T.., Mostardeiro C. P.., Da Cruz I. B.., Oliveira R. M.., Ilha V.., Dalcol I. I.., Morel A. F.PLoS One. 2014. 9:, e97000.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Activation of PPAR-α and Wnt/β-catenin by Luffa cylindrica Supercritical Carbon Dioxide Extract
- Comparative Analysis of Anticancer and Antibacterial Activities among Seven Trametes Species
- Depositional characteristics of atmospheric polybrominated diphenyl ethers on tree barks
- Analysis of α-Glucosidase Inhibitory Constituents from Acer tegmentosum Using LC-QTOF MS/MS And Molecular Networking
- Optimization of the Extraction Process for Bioactive Compounds from the Root Barks of Moringa oleifera