Korean J Pain.
2017 Oct;30(4):272-280. 10.3344/kjp.2017.30.4.272.
Retrospective analysis of the financial break-even point for intrathecal morphine pump use in Korea
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Seoul National University Hospital College of Medicine, Seoul, Korea. jymoon0901@gmail.com
- 2Department of Anesthesiology and Pain Medicine, National Police Hospital, Seoul, Korea.
- 3Integrated Cancer Management Center, Seoul National University Cancer Hospital, Seoul, Korea.
- KMID: 2392904
- DOI: http://doi.org/10.3344/kjp.2017.30.4.272
Abstract
- BACKGROUND
The high cost of intrathecal morphine pump (ITMP) implantation may be the main obstacle to its use. Since July 2014, the Korean national health insurance (NHI) program began paying 50% of the ITMP implantation cost in select refractory chronic pain patients. The aims of this study were to investigate the financial break-even point and patients' satisfaction in patients with ITMP treatment after the initiation of the NHI reimbursement.
METHODS
We collected data retrospectively or via direct phone calls to patients who underwent ITMP implantation at a single university-based tertiary hospital between July 2014 and May 2016. Pain severity, changes in the morphine equivalent daily dosage (MEDD), any adverse events, and patients' satisfaction were determined. We calculated the financial break-even point of ITMP implantation via investigating the patient's actual medical costs and insurance information.
RESULTS
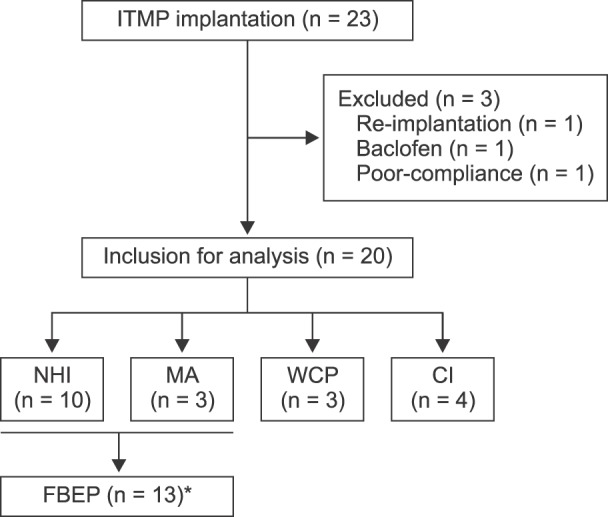
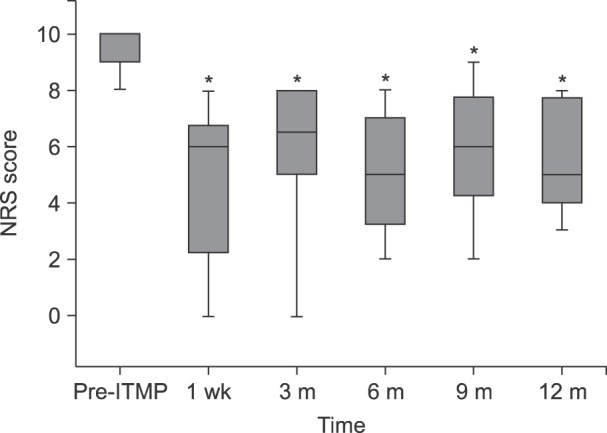
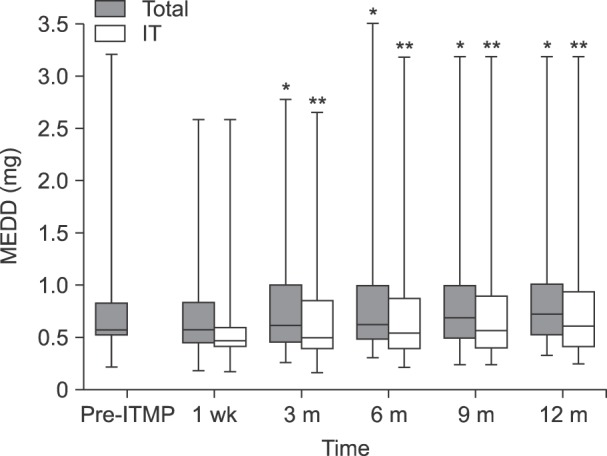
During the studied period, 23 patients received ITMP implantation, and 20 patients were included in our study. Scores on an 11-point numeric rating scale (NRS) for pain were significantly reduced compared to the baseline value (P < 0.001). The MEDD before ITMP implantation was 0.59 [IQR: 0.55-0.82]. The total MEDD increased steadily to 0.77 [IQR: 0.53-1.08] at 1 year, which was 126% of the baseline (P < 0.001). More than a half (60%) responded that the ITMP therapy was somewhat satisfying. The financial break-even point was 28 months for ITMP treatment after the NHI reimbursement policy.
CONCLUSIONS
ITMP provided effective chronic pain management with improved satisfaction and reasonable financial break-even point of 28 months with 50% financial coverage by NHI program.
Keyword
MeSH Terms
Figure
Reference
-
1. Cousins MJ, Brennan F, Carr DB. Pain relief: a universal human right. Pain. 2004; 112:1–4. PMID: 15494176.
Article2. Bolash R, Udeh B, Saweris Y, Guirguis M, Dalton JE, Makarova N, et al. Longevity and cost of implantable intrathecal drug delivery systems for chronic pain management: a retrospective analysis of 365 patients. Neuromodulation. 2015; 18:150–155. PMID: 25250852.
Article3. Goldberg DS, McGee SJ. Pain as a global public health priority. BMC Public Health. 2011; 11:770. PMID: 21978149.
Article4. Breivik H, Eisenberg E, OBrien T. OPENMinds. The individual and societal burden of chronic pain in Europe: the case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Public Health. 2013; 13:1229. PMID: 24365383.
Article5. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006; 10:287–333. PMID: 16095934.
Article6. Smith BH, Torrance N. Management of chronic pain in primary care. Curr Opin Support Palliat Care. 2011; 5:137–142. PMID: 21415754.
Article7. Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic non-malignant pain: systematic review of randomised trials of oral opioids. Arthritis Res Ther. 2005; 7:R1046–R1051. PMID: 16207320.8. Wallace M, Yaksh TL. Long-term spinal analgesic delivery: a review of the preclinical and clinical literature. Reg Anesth Pain Med. 2000; 25:117–157. PMID: 10746527.
Article9. Anderson VC, Burchiel KJ. A prospective study of long-term intrathecal morphine in the management of chronic nonmalignant pain. Neurosurgery. 1999; 44:289–300. PMID: 9932882.
Article10. Paice JA, Winkelmüller W, Burchiel K, Racz GB, Prager JP. Clinical realities and economic considerations: efficacy of intrathecal pain therapy. J Pain Symptom Manage. 1997; 14:S14–S26. PMID: 9291707.
Article11. Deer TR, Krames E, Levy RM, Hassenbusch SJ 3rd, Prager JP. Practice choices and challenges in the current intrathecal therapy environment: an online survey. Pain Med. 2009; 10:304–309. PMID: 19254334.
Article12. Kim EJ, Moon JY, Kim YC, Park KS, Yoo YJ. Intrathecal morphine infusion therapy in management of chronic pain: present and future implementation in Korea. Yonsei Med J. 2016; 57:475–481. PMID: 26847303.
Article13. The Korean Pain Society. Textbook of pain medicine. 4th ed. Seoul: Shinwon Medical;2012. p. 469–480.14. Tracey I, Bushnell MC. How neuroimaging studies have challenged us to rethink: is chronic pain a disease? J Pain. 2009; 10:1113–1120. PMID: 19878862.
Article15. Hamza M, Doleys D, Wells M, Weisbein J, Hoff J, Martin M, et al. Prospective study of 3-year follow-up of low-dose intrathecal opioids in the management of chronic nonmalignant pain. Pain Med. 2012; 13:1304–1313. PMID: 22845187.
Article16. Auld AW, Maki-Jokela A, Murdoch DM. Intraspinal narcotic analgesia in the treatment of chronic pain. Spine (Phila Pa 1976). 1985; 10:777–781. PMID: 3841231.
Article17. Turner JA, Sears JM, Loeser JD. Programmable intrathecal opioid delivery systems for chronic noncancer pain: a systematic review of effectiveness and complications. Clin J Pain. 2007; 23:180–195. PMID: 17237668.
Article18. Duarte RV, Raphael JH, Haque MS, Southall JL, Ashford RL. A predictive model for intrathecal opioid dose escalation for chronic non-cancer pain. Pain Physician. 2012; 15:363–369. PMID: 22996848.19. Atli A, Theodore BR, Turk DC, Loeser JD. Intrathecal opioid therapy for chronic nonmalignant pain: a retrospective cohort study with 3-year follow-up. Pain Med. 2010; 11:1010–1016. PMID: 20492572.
Article20. Kumar K, Kelly M, Pirlot T. Continuous intrathecal morphine treatment for chronic pain of nonmalignant etiology: long-term benefits and efficacy. Surg Neurol. 2001; 55:79–86. PMID: 11301086.
Article21. Grider JS, Harned ME, Etscheidt MA. Patient selection and outcomes using a low-dose intrathecal opioid trialing method for chronic nonmalignant pain. Pain Physician. 2011; 14:343–351. PMID: 21785477.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Implantable Intrathecal Drug Delivery Pump in Complex Regional Pain Syndrome Patient : A case report
- Intrathecal Morphine Infusion Therapy in Management of Chronic Pain: Present and Future Implementation in Korea
- Repetitive single subarachnoid injections for trial administration of the intrathecal morphine pump in patients with intractable non-cancer pain: A case report
- Treatment of Urinary Rstsntion due to Intrathecal Injection of Morphine
- Intrathecal baclofen pump implantation for complex regional pain syndrome in a patient with a spinal cord stimulator: consideration about optimal location of intrathecal catheter tip: A case report