J Periodontal Implant Sci.
2017 Oct;47(5):292-311. 10.5051/jpis.2017.47.5.292.
Targeting the epitope spreader Pep19 by naïve human CD45RA⺠regulatory T cells dictates a distinct suppressive T cell fate in a novel form of immunotherapy
- Affiliations
-
- 1Department of Periodontology, Dental Research Institute, Pusan National University Dental Hospital, Pusan National University School of Dentistry, Yangsan, Korea. jrapa@pusan.ac.kr
- 2Molecular Recognition Research Center, Korea Institute of Science and Technology, Seoul, Korea.
- 3Department of Pharmacology, Pusan National University School of Medicine, Yangsan, Korea.
- KMID: 2392717
- DOI: http://doi.org/10.5051/jpis.2017.47.5.292
Abstract
- PURPOSE
Beyond the limited scope of non-specific polyclonal regulatory T cell (Treg)-based immunotherapy, which depends largely on serendipity, the present study explored a target Treg subset appropriate for the delivery of a novel epitope spreader Pep19 antigen as part of a sophisticated form of immunotherapy with defined antigen specificity that induces immune tolerance.
METHODS
Human polyclonal CD4âºCD25âºCD127(lo−) Tregs (127-Tregs) and naïve CD4âºCD25âºCD45RA⺠Tregs (45RA-Tregs) were isolated and were stimulated with target peptide 19 (Pep19)-pulsed dendritic cells in a tolerogenic milieu followed by ex vivo expansion. Low-dose interleukin-2 (IL-2) and rapamycin were added to selectively exclude the outgrowth of contaminating effector T cells (Teffs). The following parameters were investigated in the expanded antigen-specific Tregs: the distinct expression of the immunosuppressive Treg marker Foxp3, epigenetic stability (demethylation in the Treg-specific demethylated region), the suppression of Teffs, expression of the homing receptors CD62L/CCR7, and CD95L-mediated apoptosis. The expanded Tregs were adoptively transferred into an NOD/scid/IL-2Rγ(−/−) mouse model of collagen-induced arthritis.
RESULTS
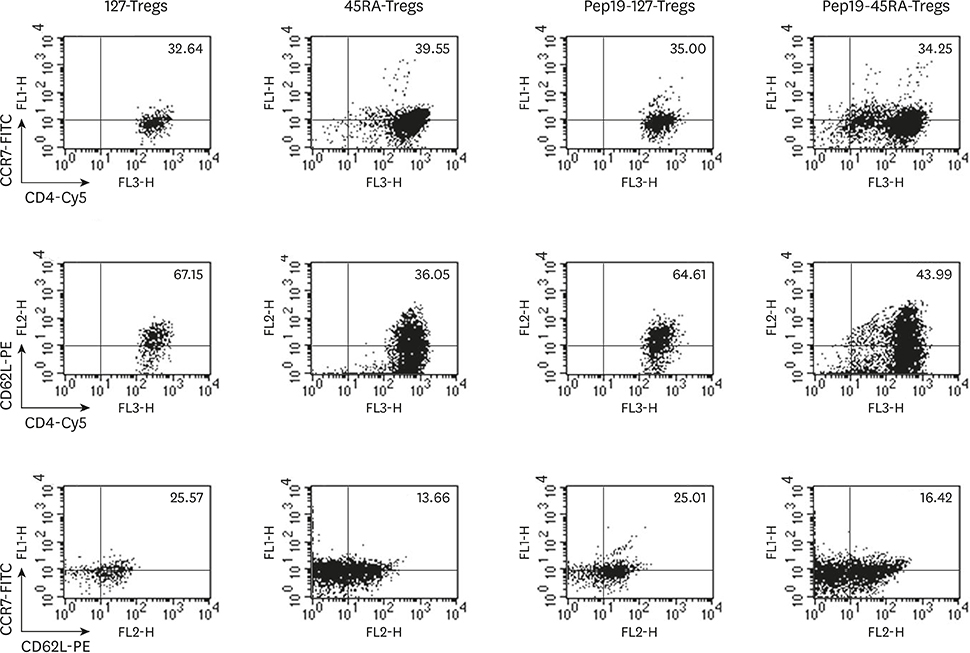
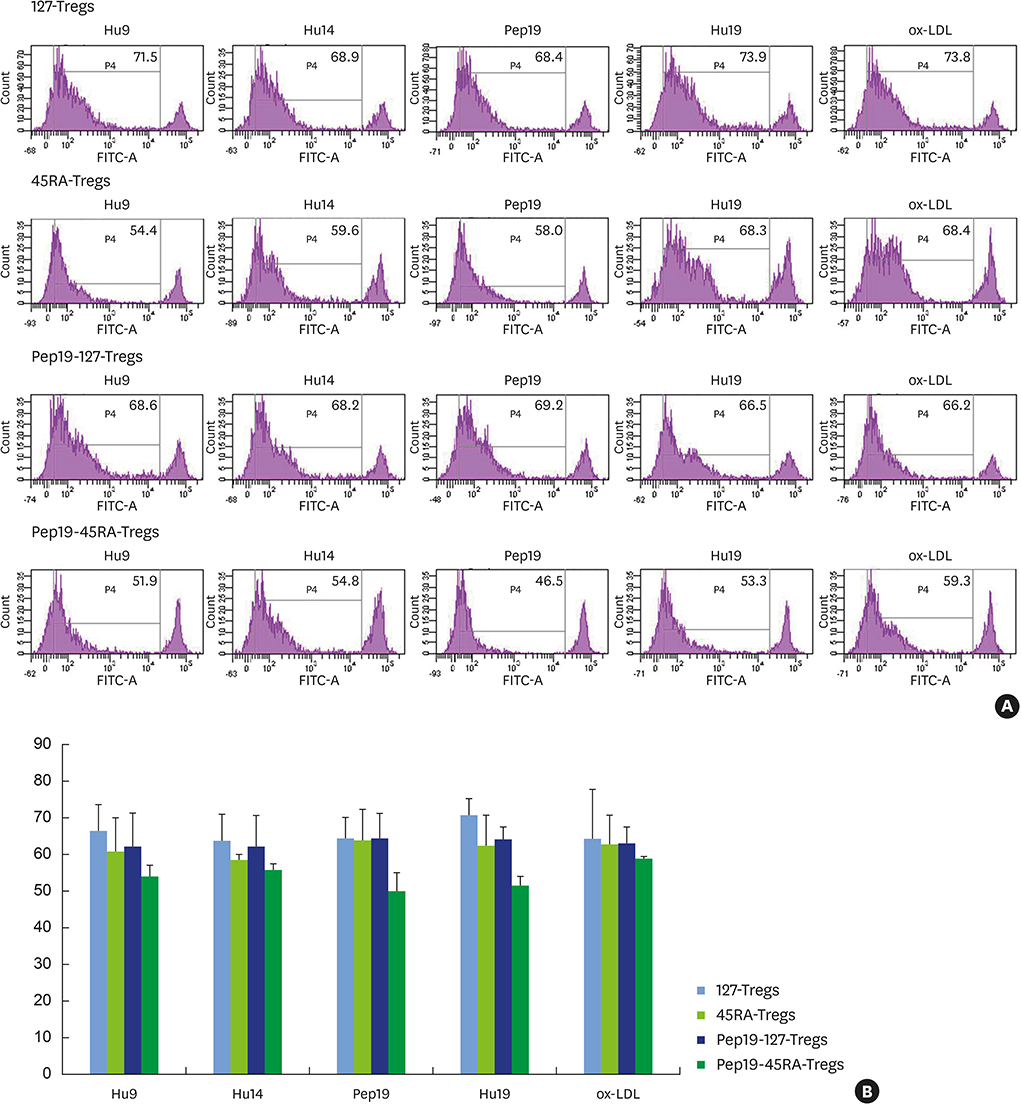
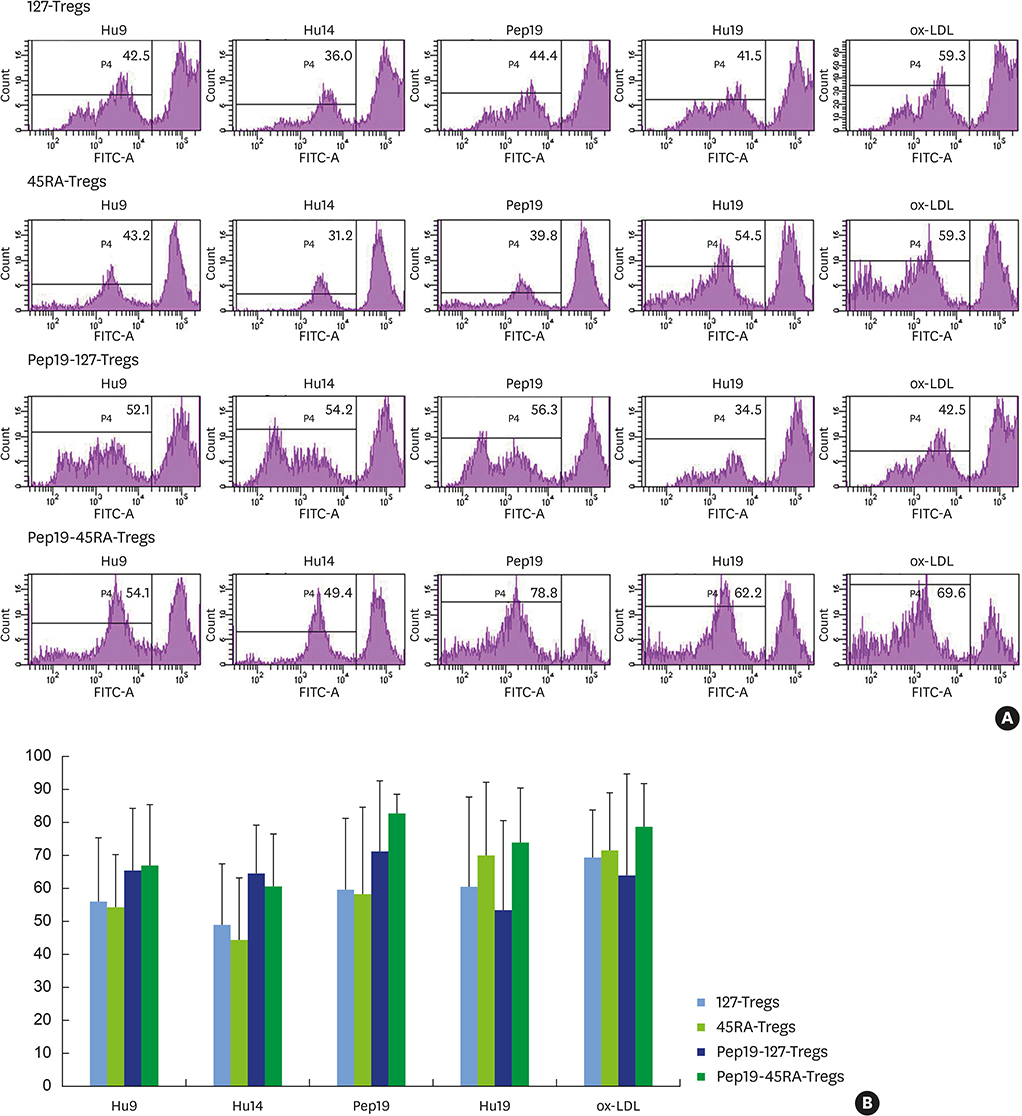
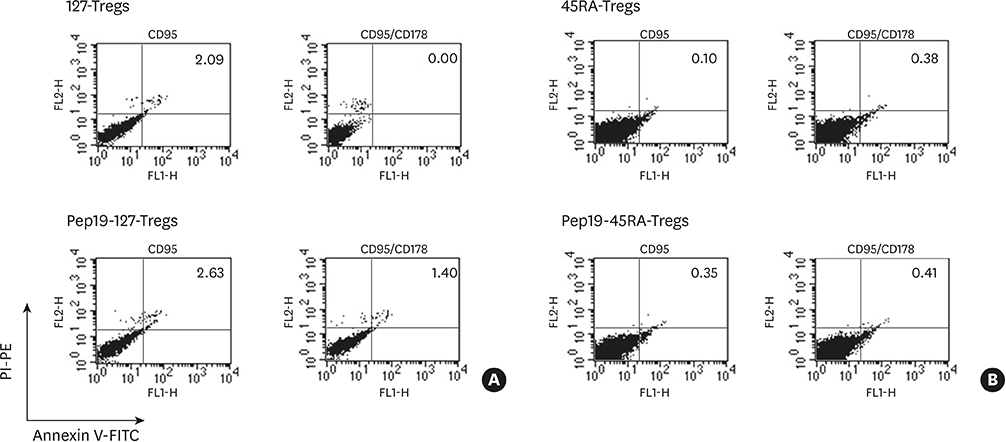
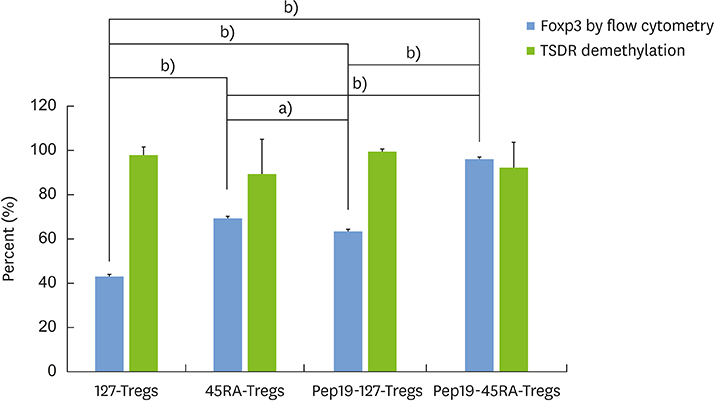
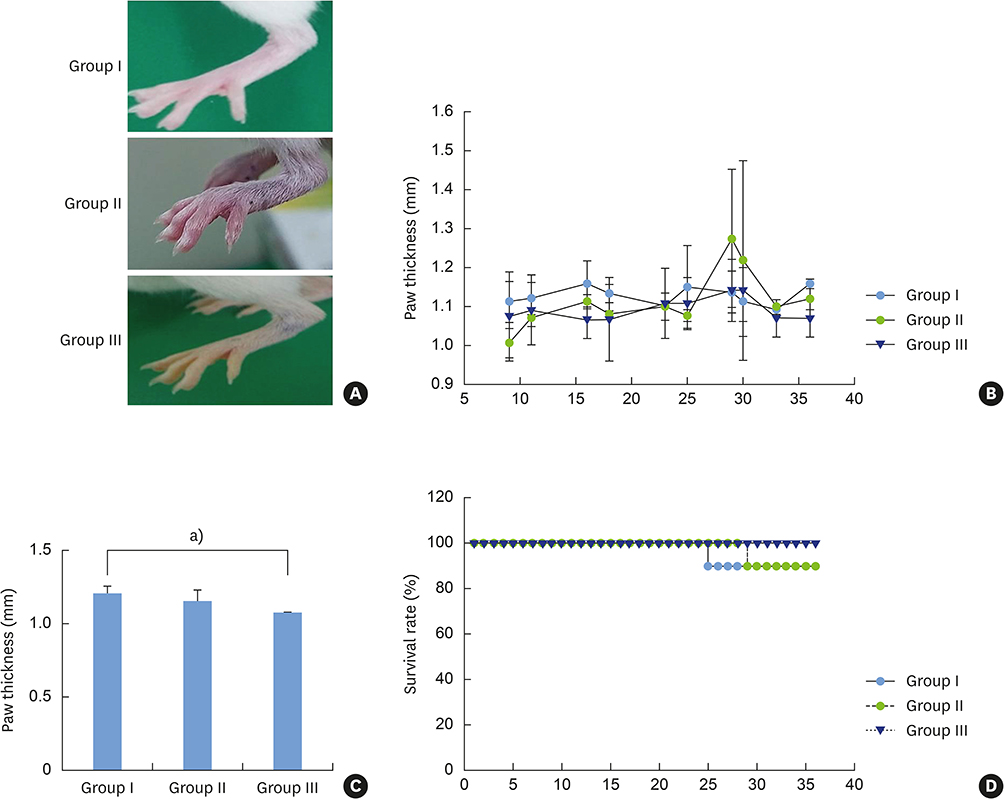
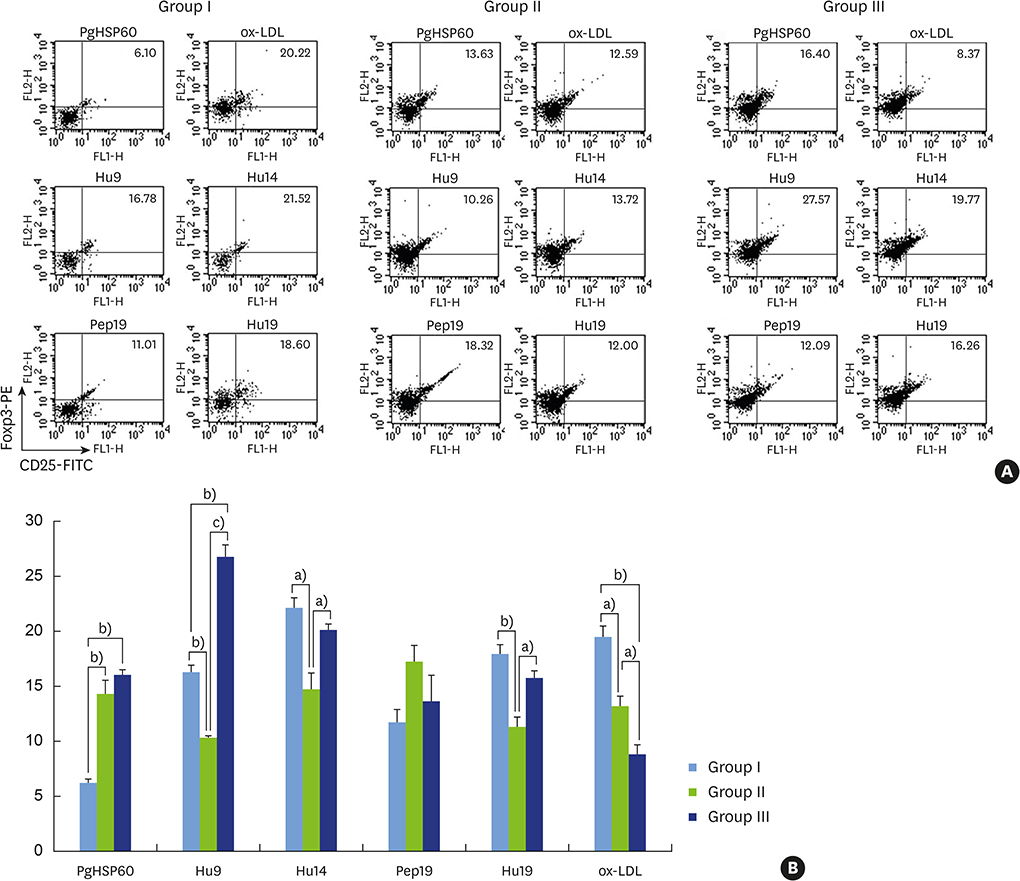
Epitope-spreader Pep19 targeting by 45RA-Tregs led to an outstanding in vitro suppressive T cell fate characterized by robust ex vivo expansion, the salient expression of Foxp3, high epigenetic stability, enhanced T cell suppression, modest expression of CD62L/CCR7, and higher resistance to CD95L-mediated apoptosis. After adoptive transfer, the distinct fate of these T cells demonstrated a potent in vivo immunotherapeutic capability, as indicated by the complete elimination of footpad swelling, prolonged survival, minimal histopathological changes, and preferential localization of CD4âºCD25⺠Tregs at the articular joints in a mechanistic and orchestrated way.
CONCLUSIONS
We propose human naïve CD4âºCD25âºCD45RA⺠Tregs and the epitope spreader Pep19 as cellular and molecular targets for a novel antigen-specific Treg-based vaccination against collagen-induced arthritis.
Keyword
MeSH Terms
-
Adoptive Transfer
Animals
Apoptosis
Arthritis, Experimental
Arthritis, Rheumatoid
Autoimmune Diseases
Dendritic Cells
Epigenomics
Eragrostis
Heat-Shock Proteins
Humans*
Immune Tolerance
Immunotherapy*
In Vitro Techniques
Interleukin-2
Joints
Mice
Sensitivity and Specificity
Sirolimus
T-Lymphocytes
T-Lymphocytes, Regulatory*
Vaccination
Heat-Shock Proteins
Interleukin-2
Sirolimus
Figure
Reference
-
1. Morgan ME, Flierman R, van Duivenvoorde LM, Witteveen HJ, van Ewijk W, van Laar JM, et al. Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum. 2005; 52:2212–2221.
Article2. Vercoulen Y, van Teijlingen NH, de Kleer IM, Kamphuis S, Albani S, Prakken BJ. Heat shock protein 60 reactive T cells in juvenile idiopathic arthritis: what is new? Arthritis Res Ther. 2009; 11:231–240.
Article3. Rodríguez-Palmero M, Franch A, Castell M, Pelegrí C, Pérez-Cano FJ, Kleinschnitz C, et al. Effective treatment of adjuvant arthritis with a stimulatory CD28-specific monoclonal antibody. J Rheumatol. 2006; 33:110–118.4. Keijzer C, Wieten L, van Herwijnen M, van der Zee R, Van Eden W, Broere F. Heat shock proteins are therapeutic targets in autoimmune diseases and other chronic inflammatory conditions. Expert Opin Ther Targets. 2012; 16:849–857.
Article5. Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006; 12:178–180.
Article6. Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med. 2015; 7:315ra189.
Article7. Miyara M, Ito Y, Sakaguchi S. Treg-cell therapies for autoimmune rheumatic diseases. Nat Rev Rheumatol. 2014; 10:543–551.
Article8. Bluestone JA, Bour-Jordan H. Current and future immunomodulation strategies to restore tolerance in autoimmune diseases. Cold Spring Harb Perspect Biol. 2012; 4:a007542.
Article9. Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the Foxp3 transcription factor. Immunity. 2009; 30:899–911.
Article10. Hoffmann P, Eder R, Boeld TJ, Doser K, Piseshka B, Andreesen R, et al. Only the CD45RA+ subpopulation of CD4+CD25high T cells gives rise to homogeneous regulatory T-cell lines upon in vitro expansion. Blood. 2006; 108:4260–4267.
Article11. Hoffmann P, Boeld TJ, Eder R, Huehn J, Floess S, Wieczorek G, et al. Loss of Foxp3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur J Immunol. 2009; 39:1088–1097.
Article12. Fritzsching B, Oberle N, Pauly E, Geffers R, Buer J, Poschl J, et al. Naive regulatory T cells: a novel subpopulation defined by resistance toward CD95L-mediated cell death. Blood. 2006; 108:3371–3378.
Article13. McMurchy AN, Bushell A, Levings MK, Wood KJ. Moving to tolerance: clinical application of T regulatory cells. Semin Immunol. 2011; 23:304–313.
Article14. Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004; 199:1455–1465.
Article15. Bluestone JA, Trotta E, Xu D. The therapeutic potential of regulatory T cells for the treatment of autoimmune disease. Expert Opin Ther Targets. 2015; 19:1091–1103.
Article16. Fischbach MA, Bluestone JA, Lim WA. Cell-based therapeutics: the next pillar of medicine. Sci Transl Med. 2013; 5:179ps7.
Article17. Persson GR. Rheumatoid arthritis and periodontitis - inflammatory and infectious connections. Review of the literature. J Oral Microbiol. 2012; 4:11829.
Article18. Arleevskaya MI, Kravtsova OA, Lemerle J, Renaudineau Y, Tsibulkin AP. How Rheumatoid arthritis can result from provocation of the immune system by microorganisms and viruses. Front Microbiol. 2016; 7:1296.
Article19. Choi JI, Chung SW, Kang HS, Rhim BY, Park YM, Kim US, et al. Epitope mapping of Porphyromonas gingivalis heat-shock protein and human heat-shock protein in human atherosclerosis. J Dent Res. 2004; 83:936–940.
Article20. Choi J, Lee SY, Kim K, Choi BK. Identification of immunoreactive epitopes of the Porphyromonas gingivalis heat shock protein in periodontitis and atherosclerosis. J Periodontal Res. 2011; 46:240–245.
Article21. Jeong E, Lee JY, Kim SJ, Choi J. Predominant immunoreactivity of Porphyromonas gingivalis heat shock protein in autoimmune diseases. J Periodontal Res. 2012; 47:811–816.
Article22. Jeong E, Kim K, Kim JH, Cha GS, Kim SJ, Kang HS, et al. Porphyromonas gingivalis HSP60 peptides have distinct roles in the development of atherosclerosis. Mol Immunol. 2015; 63:489–496.
Article23. Kwon EY, Cha GS, Jeong E, Lee JY, Kim SJ, Surh CD, et al. Pep19 drives epitope spreading in periodontitis and periodontitis-associated autoimmune diseases. J Periodontal Res. 2016; 51:381–394.
Article24. Joo JY, Cha GS, Chung J, Lee JY, Kim SJ, Choi J. Peptide 19 of Porphyromonas gingivalis heat shock protein is a potent inducer of low-density lipoprotein oxidation. J Periodontol. 2017; 88:e58–e64.25. Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008; 118:3537–3545.
Article26. Haque M, Fino K, Lei F, Xiong X, Song J. Utilizing regulatory T cells against rheumatoid arthritis. Front Oncol. 2014; 4:209.
Article27. Zonneveld-Huijssoon E, Roord ST, de Jager W, Klein M, Albani S, Anderton SM, et al. Bystander suppression of experimental arthritis by nasal administration of a heat shock protein peptide. Ann Rheum Dis. 2011; 70:2199–2206.
Article28. Overacre AE, Vignali DA. (reg) stability: to be or not to be. Curr Opin Immunol. 2016; 39:39–43.29. Ohkura N, Hamaguchi M, Morikawa H, Sugimura K, Tanaka A, Ito Y, et al. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity. 2012; 37:785–799.
Article30. Masteller EL, Tang Q, Bluestone JA. Antigen-specific regulatory T cells--ex vivo expansion and therapeutic potential. Semin Immunol. 2006; 18:103–110.
Article31. Shevach EM. Mechanisms of Foxp3+ T regulatory cell-mediated suppression. Immunity. 2009; 30:636–645.
Article32. Grant CR, Liberal R, Mieli-Vergani G, Vergani D, Longhi MS. Regulatory T-cells in autoimmune diseases: challenges, controversies and--yet--unanswered questions. Autoimmun Rev. 2015; 14:105–116.
Article33. Schneider A, Buckner JH. Assessment of suppressive capacity by human regulatory T cells using a reproducible, bi-directional CFSE-based in vitro assay. Methods Mol Biol. 2011; 707:233–241.34. Boks MA, Zwaginga JJ, van Ham SM, ten Brinke A. An optimized CFSE-based T-cell suppression assay to evaluate the suppressive capacity of regulatory T-cells induced by human tolerogenic dendritic cells. Scand J Immunol. 2010; 72:158–168.
Article35. Venken K, Thewissen M, Hellings N, Somers V, Hensen K, Rummens JL, et al. A CFSE based assay for measuring CD4+CD25+ regulatory T cell mediated suppression of auto-antigen specific and polyclonal T cell responses. J Immunol Methods. 2007; 322:1–11.
Article36. Raker VK, Domogalla MP, Steinbrink K. Tolerogenic dendritic cells for regulatory T cell induction in man. Front Immunol. 2015; 6:569.
Article37. Trzonkowski P, Bacchetta R, Battaglia M, Berglund D, Bohnenkamp HR, ten Brinke A, et al. Hurdles in therapy with regulatory T cells. Sci Transl Med. 2015; 7:304ps18.
Article38. Brusko TM, Koya RC, Zhu S, Lee MR, Putnam AL, McClymont SA, et al. Human antigen-specific regulatory T cells generated by T cell receptor gene transfer. PLoS One. 2010; 5:e11726.
Article39. Gratz IK, Campbell DJ. Organ-specific and memory Treg cells: specificity, development, function, and maintenance. Front Immunol. 2014; 5:333.
Article40. Chaudhry A, Rudensky AY. Control of inflammation by integration of environmental cues by regulatory T cells. J Clin Invest. 2013; 123:939–944.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Robust immunoreactivity of teenager sera against peptide 19 from Porphyromonas gingivalis HSP60
- T Cell's Sense of Self: a Role of Self-Recognition in Shaping Functional Competence of Naïve T Cells
- Ribavirin Does Not Impair the Suppressive Activity of Foxp3+CD4+CD25+ Regulatory T Cells
- The Role of Regulatory T Cells in Cancer
- Immuno-oncology for B-cell lymphomas