Nutr Res Pract.
2009 Dec;3(4):259-264.
Loquat (Eriobotrya japonica) extracts suppress the adhesion, migration and invasion of human breast cancer cell line
- Affiliations
-
- 1Major in Food & Nutrition, Mokpo National University, 61 Dorim-ri cheonggye-myeon, Muan-gun, Jeonnam 534-729, Korea. kha@mokpo.ac.kr
- 2Major in Medical Plant Resources, Mokpo National University, 61 Dorim-ri cheonggye-myeon, Muan-gun, Jeonnam 534-729, Korea.
- 3Korea INSpharm Research institute, 1065 Daepo-ri, Dong-myeon, Hwasun-gun, Jeonnam 519-882, Korea.
Abstract
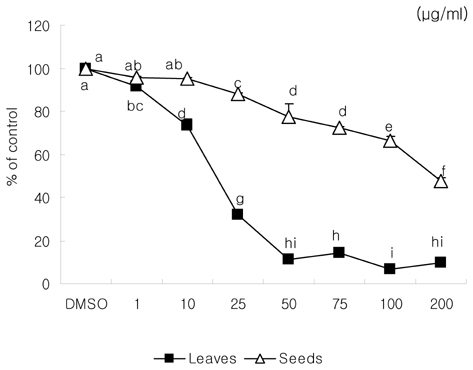
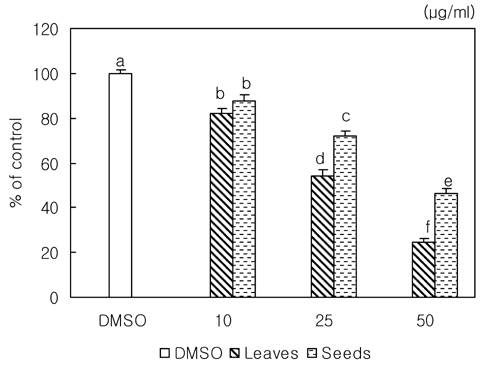
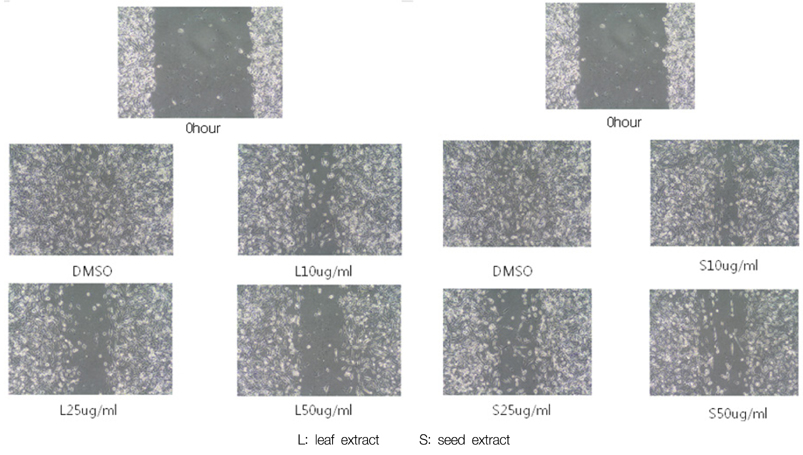
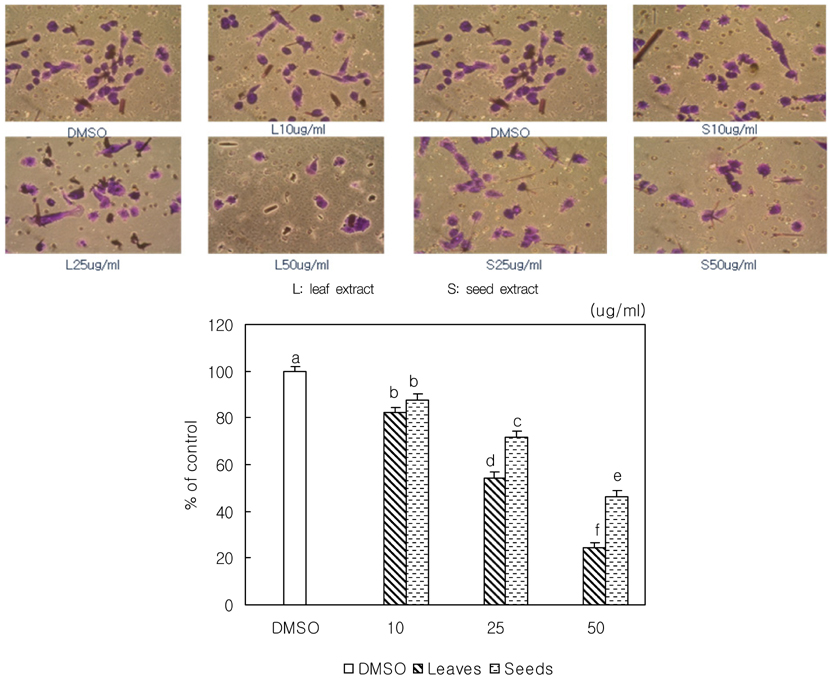
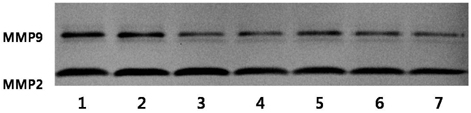
- We examined the inhibitory effects of loquat methanol extract on the adhesion, migration, invasion and matrix metalloproteinase (MMP) activities of MDA-MB-231 human breast cancer cell line. Cells were cultured with DMSO or with 10, 25, or 50 microg/ml of loquat methanol extract. Both leaf and seed extracts significantly inhibited growth of MDA-MB-231 cells in a dose-dependent manner, although leaf extract was more effective. Adhesion and migration were significantly inhibited by loquat extracts in a dose-dependent manner. Loquat extract also inhibited the invasion of breast cancer cells in a dose-dependent manner and leaf extract was more effective than seed extract. MMP-2 and MMP-9 activities were also inhibited by loquat extract. Our results indicate that methanol extracts of loquat inhibit the adhesion, migration and invasion of human breast cancer cells partially through the inhibition of MMP activity and leaf extract has more anti-metastatic effects in cell based assay than seed extract. Clinical application of loquat extract as a potent chemopreventive agent may be helpful in limiting breast cancer invasion and metastasis.
Keyword
MeSH Terms
Figure
Reference
-
1. Azios NG, Dharmawardhane SF. Resveratrol and estradiol exert disparate effects on cell migration, cell surface acting structures and focal adhesion assembly in MDA-MB-231 human breast cancer cells. Neoplasia. 2005. 7:128–140.
Article2. Banno N, Akihisa T, Tokuda H, Yasukawa K, Taguchi Y, Akazawa H, Ukiya M, Kinura Y, Suxuki T, Nishino H. Anti-inflammatory and antitumor-promotin effects of the triterpene acids from the leaves of Eriobotrya japonica. Biol Pharm Bull. 2005. 25:1995–1999.3. Cos S, Fernández R, Güézmes A, Sánchez-Barceló EJ. Influence of melatonin on invasive and metastatic properties of MCF-7 human breast cancer cells. Cancer Res. 1998. 58:4383–4390.4. Festuccia C, Bologna M, Gravina GL, Guerra F, Angelucci A, Villanova I, Millimaggi D, Teti A. Osteoblast conditioned media contain TGF-β and modulate the migration of prostate tumor cells and their interactions with extracellular matrix component. Int J Cancer. 1999. 81:395–403.
Article5. Festuccia C, Guerra F, D'Acenzo S, Guinciuglio D, Albini A, Bologna M. In vitro regulation of pericellular proteolysis in prostatic tumor cells treated with bombesin. Int J Cancer. 1998. 75:418–431.
Article6. Goodman SL, Vollmer HP, Birchmeier W. Control of cell locomotion: pertubation with an antibody directed against specific glycoproteins. Cell. 1985. 41:1029–1038.
Article7. Hazan RB, Phillips GR, Qiao RF, Norton L, Aaeonson SA. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol. 2000. 148:779–790.
Article8. Huang Y, Kim MS, Ratovitski E. Midkine promote tetraspanin-integrin interaction and inuceds FAK-Stat1 alpha pathway contributing to migration/invasivemess of human head and neck squamous cell carcinoma cells. Biochem Biophys Res Commun. 2008. 377:474–478.
Article9. Ito H, Kobayashi E, Takamatsu Y, Li S, Hatano T, Sakagami H, Kusama K, Satoh K, Sugita D, Shimura S, Itoh Y, Yoshida T. Polyphenols from Eribotrya japonica and their cytotoxicity against human oral cancer cell lines. Chem Pharm Bull. 2000. 48:687–693.
Article10. Johansson N, Ahonen M, Kajari VM. Matrix metalliproteinases in tumor invasion. Cell Mol Life Sci. 2000. 57:5–15.11. Kawasaki G, Yanamoto S, Yoshitomi I, Yamada S, Mizuno A. Overexpression of metastasis-associated MTA1 in oral squamous cell carcinomas:correlation with metastasis and invasion. Int J Oral Maxillofac Surg. 2008. 37:1039–1046.
Article12. Kim E, Kim MS, Rhyu DY, Min OJ, Baek HY, Kim YJ, Kim HA. Hypoglycemic effect of Eryobotrya japonica (E. japonica) in db/db mice. The Korean Journal of Food and Nutrition. 2009. 22:159–165.13. Kohn EC, Liotta LA. Molecular insights into cancer invasion: strategies for prevention and intervention. Cancer Res. 1995. 55:1856–1862.14. Liang ZZ, Aquino R, Feo VD, Shimone FD, Pizza C. Polyhaydroxylated triterpene from Eribotrya japonica. Planta Med. 1990. 56:330–332.15. Meng Q, Goldberf ID, Rosen EM, Fan S. Inhibitory effects of indole-3-carbinol on invasion and migration in human breast cancer cells. Breast Cancer Res Treat. 2000. 63:147–152.
Article16. Nozato N, Matsumoto K, Uemitsu N. Triterpene from the leaves of Eribotrya japonica. Nat Med (Tokyo). 1994. 48:336.17. Shaikh FM, Seales EC, Clem WC, Hennessy KM, Zhuo Y, Bellis SL. Tumor cell migration and invasion are regulated by expression of variant integrin glycoforms. Exp Cell Res. 2008. 314:2941–2950.
Article18. Shimizu M, Uemitsu N, Shirota M, Matsumoto K, Tezuka U. A new triterpene ester from Eribotrya japonica. Chem Pharm Bull. 1996. 44:2181–2182.19. Song G, Ohashi T, Sakamoto N, Sato M. Adhesive force of human hepatoma HepG2 cells to endothelial cells and expression of E-selectin. Mol Cell Biomech. 2006. 3:61–68.20. Sun Y, Lu N, Ling Y, Gao Y, Chen Y, Wang L, Hu R, Qi Q, Liu W, Yang Y, You Q, Guo Q. Oroxylin A suppress invasion through down-regulating the expression of matrix metallic proteinase-2/9 in MDA-MB-435 human breast cancer cells. Eur J Pharmacol. 2009. 603:22–28.
Article21. Tommasi ND, Shimone FD, Pizza C. Cinstituents of Eribotrya japonica : a study of their antiviral properties. J Nat Prod. 1992. 55:1067–1073.22. Weber GF. Molecular mechanism of metastasis. Cancer Lett. 2008. 270:181–190.23. Yoneda T, Sasaki A, Mundy GR. Osteolytic bone metastasis in breast cancer. Breast Cancer Res Treat. 1994. 32:73–84.
Article24. Zheng DQ, Woodard AS, Fronaro M, Tallini G, Languino LR. Prostatic Carcinoma Cell Migration Via αvβ3 integrin is modulated by a focal adhesion kinase pathway. Cancer Res. 1999. 59:1655–1664.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Loquat (Eriobotrya japonica) leaf extract inhibits the growth of MDA-MB-231 tumors in nude mouse xenografts and invasion of MDA-MB-231 cells
- Inhibitory effect of Erythronium japonicum on the human breast cancer cell metastasis
- Effect of Curcumin on Cancer Invasion and Matrix Metalloproteinase-9 Activity in MDA-MB-231 Human Breast Cancer Cell
- Effects of Epigallocatechin Gallate on Adhesion, Invasion and Matrix Metalloproteinase Activity in MDA-MB-231 Human Breast Cancer Cells
- The Anti-tumor Effect of Macrolides on the Extracellular Matrix Invasion and Cell Adhesion in Human Non-small Cell Lung Cancer Cells