Cancer Res Treat.
2017 Jul;49(3):635-642. 10.4143/crt.2016.282.
Nomograms Predicting Platinum Sensitivity, Progression-Free Survival, and Overall Survival Using Pretreatment Complete Blood Cell Counts in Epithelial Ovarian Cancer
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. ds123.bae@samsung.com
- 2Biostatistics and Clinical Epidemiology Center, Samsung Medical Center, Seoul, Korea.
- 3Division of Gynecologic Oncology, Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada. heyu0a@gmail.com
- KMID: 2388309
- DOI: http://doi.org/10.4143/crt.2016.282
Abstract
- PURPOSE
This study was conducted to evaluate the prognostic significance of pre-treatment complete blood cell count (CBC), including white blood cell (WBC) differential, in epithelial ovarian cancer (EOC) patients with primary debulking surgery (PDS) and to develop nomograms for platinum sensitivity, progression-free survival (PFS), and overall survival (OS).
MATERIALS AND METHODS
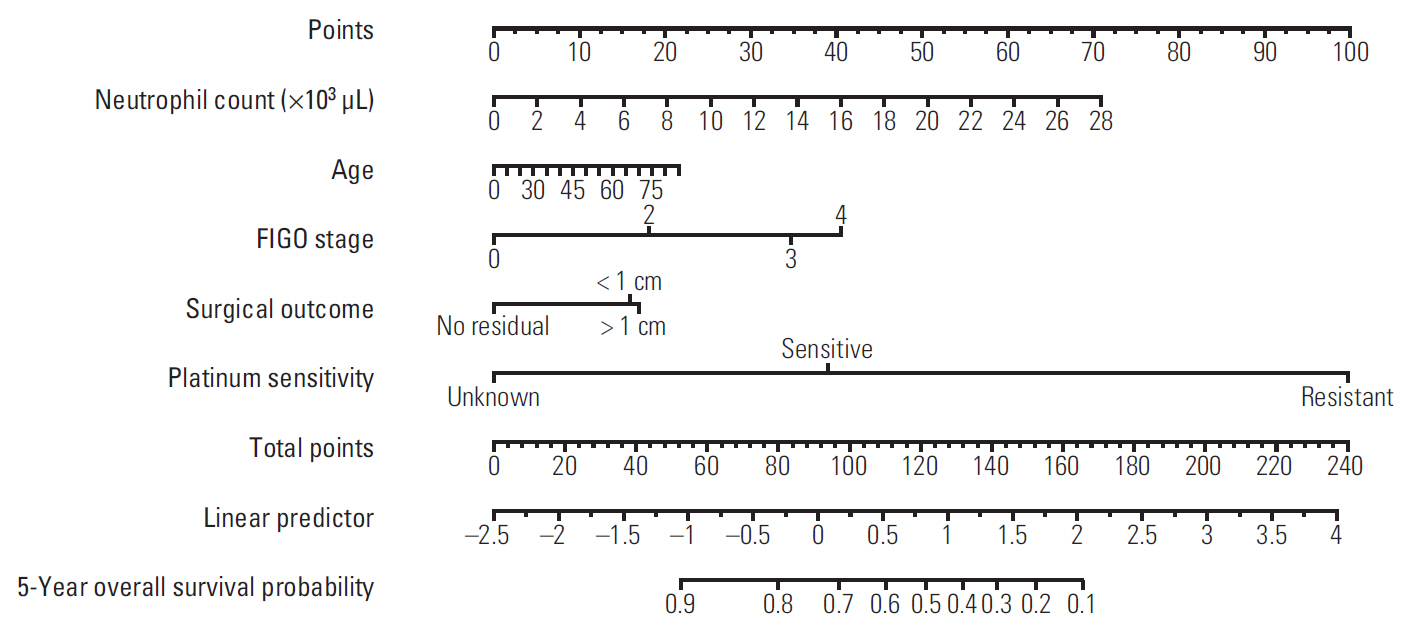
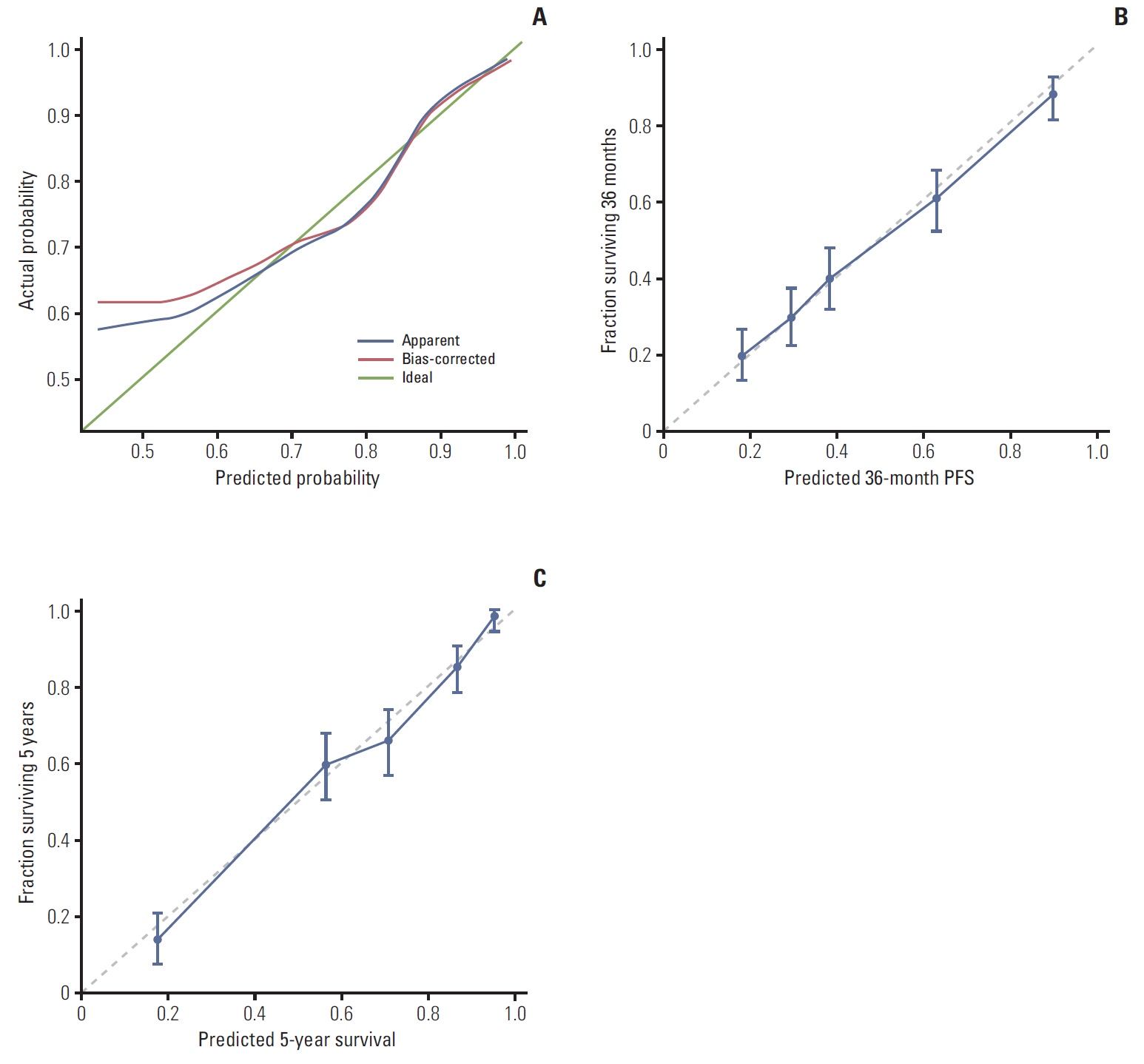
We retrospectively reviewed the records of 757 patients with EOC whose primary treatment consisted of surgical debulking and chemotherapy at Samsung Medical Center from 2002 to 2012. We subsequently created nomograms for platinum sensitivity, 3-year PFS, and 5-year OS as prediction models for prognostic variables including age, stage, grade, cancer antigen 125 level, residual disease after PDS, and pre-treatment WBC differential counts. The models were then validated by 10-fold cross-validation (CV).
RESULTS
In addition to stage and residual disease after PDS, which are known predictors, lymphocyte and monocyte count were found to be significant prognostic factors for platinum-sensitivity, platelet count for PFS, and neutrophil count for OS on multivariate analysis. The area under the curves of platinum sensitivity, 3-year PFS, and 5-year OS calculated by the 10-fold CV procedure were 0.7405, 0.8159, and 0.815, respectively.
CONCLUSION
Prognostic factors including pre-treatment CBC were used to develop nomograms for platinum sensitivity, 3-year PFS, and 5-year OS of patients with EOC. These nomograms can be used to better estimate individual outcomes.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Development of Web-Based Nomograms to Predict Treatment Response and Prognosis of Epithelial Ovarian Cancer
Se Ik Kim, Minsun Song, Suhyun Hwangbo, Sungyoung Lee, Untack Cho, Ju-Hyun Kim, Maria Lee, Hee Seung Kim, Hyun Hoon Chung, Dae-Shik Suh, Taesung Park, Yong-Sang Song
Cancer Res Treat. 2019;51(3):1144-1155. doi: 10.4143/crt.2018.508.
Reference
-
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015; 65:5–29.
Article2. Vaidya AP, Curtin JP. The follow-up of ovarian cancer. Semin Oncol. 2003; 30:401–12.
Article3. Cotte E, Glehen O, Mohamed F, Lamy F, Falandry C, Golfier F, et al. Cytoreductive surgery and intraperitoneal chemohyperthermia for chemo-resistant and recurrent advanced epithelial ovarian cancer: prospective study of 81 patients. World J Surg. 2007; 31:1813–20.
Article4. Markman M, Rothman R, Hakes T, Reichman B, Hoskins W, Rubin S, et al. Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol. 1991; 9:389–93.
Article5. Hirashima K, Watanabe M, Shigaki H, Imamura Y, Ida S, Iwatsuki M, et al. Prognostic significance of the modified Glasgow prognostic score in elderly patients with gastric cancer. J Gastroenterol. 2014; 49:1040–6.
Article6. Oh BS, Jang JW, Kwon JH, You CR, Chung KW, Kay CS, et al. Prognostic value of C-reactive protein and neutrophil-to-lymphocyte ratio in patients with hepatocellular carcinoma. BMC Cancer. 2013; 13:78.
Article7. Bishara S, Griffin M, Cargill A, Bali A, Gore ME, Kaye SB, et al. Pre-treatment white blood cell subtypes as prognostic indicators in ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 2008; 138:71–5.
Article8. Grant S, Chen YQ, May S. Performance of goodness-of-fit tests for the Cox proportional hazards model with time-varying covariates. Lifetime Data Anal. 2014; 20:355–68.
Article9. Simon RM, Subramanian J, Li MC, Menezes S. Using cross-validation to evaluate predictive accuracy of survival risk classifiers based on high-dimensional data. Brief Bioinform. 2011; 12:203–14.
Article10. Youden WJ. Index for rating diagnostic tests. Cancer. 1950; 3:32–5.
Article11. Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000; 56:337–44.
Article12. Sorbellini M, Kattan MW, Snyder ME, Reuter V, Motzer R, Goetzl M, et al. A postoperative prognostic nomogram predicting recurrence for patients with conventional clear cell renal cell carcinoma. J Urol. 2005; 173:48–51.
Article13. Chi DS, Palayekar MJ, Sonoda Y, Abu-Rustum NR, Awtrey CS, Huh J, et al. Nomogram for survival after primary surgery for bulky stage IIIC ovarian carcinoma. Gynecol Oncol. 2008; 108:191–4.
Article14. Shariat SF, Karakiewicz PI, Suardi N, Kattan MW. Comparison of nomograms with other methods for predicting outcomes in prostate cancer: a critical analysis of the literature. Clin Cancer Res. 2008; 14:4400–7.
Article15. Cohen JG, Tran AQ, Rimel BJ, Cass I, Walsh CS, Karlan BY, et al. Thrombocytosis at secondary cytoreduction for recurrent ovarian cancer predicts suboptimal resection and poor survival. Gynecol Oncol. 2014; 132:556–9.
Article16. Cho H, Hur HW, Kim SW, Kim SH, Kim JH, Kim YT, et al. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother. 2009; 58:15–23.
Article17. Davis AN, Afshar-Kharghan V, Sood AK. Platelet effects on ovarian cancer. Semin Oncol. 2014; 41:378–84.
Article18. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008; 454:436–44.
Article19. Milne K, Alexander C, Webb JR, Sun W, Dillon K, Kalloger SE, et al. Absolute lymphocyte count is associated with survival in ovarian cancer independent of tumor-infiltrating lymphocytes. J Transl Med. 2012; 10:33.
Article20. Williams KA, Labidi-Galy SI, Terry KL, Vitonis AF, Welch WR, Goodman A, et al. Prognostic significance and predictors of the neutrophil-to-lymphocyte ratio in ovarian cancer. Gynecol Oncol. 2014; 132:542–50.
Article21. Armaiz-Pena GN, Gonzalez-Villasana V, Nagaraja AS, Rodriguez-Aguayo C, Sadaoui NC, Stone RL, et al. Adrenergic regulation of monocyte chemotactic protein 1 leads to enhanced macrophage recruitment and ovarian carcinoma growth. Oncotarget. 2015; 6:4266–73.
Article22. Stone RL, Nick AM, McNeish IA, Balkwill F, Han HD, Bottsford-Miller J, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012; 366:610–8.23. Clark TG, Stewart M, Rye T, Smyth JF, Gourley C. Validation of a new prognostic index for advanced epithelial ovarian cancer: results from its application to a UK-based cohort. J Clin Oncol. 2007; 25:5669–70.
Article24. Barlin JN, Yu C, Hill EK, Zivanovic O, Kolev V, Levine DA, et al. Nomogram for predicting 5-year disease-specific mortality after primary surgery for epithelial ovarian cancer. Gynecol Oncol. 2012; 125:25–30.
Article25. Rutten MJ, Boldingh JH, Schuit E, Trum H, van Driel W, Mol BW, et al. Development and internal validation of a prognostic model for survival after debulking surgery for epithelial ovarian cancer. Gynecol Oncol. 2014; 135:13–8.
Article26. Gerestein CG, Eijkemans MJ, de Jong D, van der Burg ME, Dykgraaf RH, Kooi GS, et al. The prediction of progression-free and overall survival in women with an advanced stage of epithelial ovarian carcinoma. BJOG. 2009; 116:372–80.
Article27. Lee CK, Simes RJ, Brown C, Gebski V, Pfisterer J, Swart AM, et al. A prognostic nomogram to predict overall survival in patients with platinum-sensitive recurrent ovarian cancer. Ann Oncol. 2013; 24:937–43.
Article28. Lee CK, Simes RJ, Brown C, Lord S, Wagner U, Plante M, et al. Prognostic nomogram to predict progression-free survival in patients with platinum-sensitive recurrent ovarian cancer. Br J Cancer. 2011; 105:1144–50.
Article29. Teramukai S, Ochiai K, Tada H, Fukushima M; Japan Multinational Trial Organization OC01-01. PIEPOC: a new prognostic index for advanced epithelial ovarian cancer: Japan Multinational Trial Organization OC01-01. J Clin Oncol. 2007; 25:3302–6.30. Bodurka DC, Deavers MT, Tian C, Sun CC, Malpica A, Coleman RL, et al. Reclassification of serous ovarian carcinoma by a 2-tier system: a Gynecologic Oncology Group Study. Cancer. 2012; 118:3087–94.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development of Web-Based Nomograms to Predict Treatment Response and Prognosis of Epithelial Ovarian Cancer
- Thrombocytosis as a prognostic marker in stage III and IV serous ovarian cancer
- An overview of the current debate between using minimally invasive surgery versus laparotomy for interval cytoreductive surgery in epithelial ovarian cancer
- Prognostic value of preoperative lymphocyte-monocyte ratio in elderly patients with advanced epithelial ovarian cancer
- Stage IIIC epithelial ovarian cancer classified solely by lymph node metastasis has a more favorable prognosis than other types of stage IIIC epithelial ovarian cancer