Ann Dermatol.
2016 Oct;28(5):555-561. 10.5021/ad.2016.28.5.555.
Kojic Acid Peptide: A New Compound with Anti-Tyrosinase Potential
- Affiliations
-
- 1Department of Biological Engineering, Inha University, Incheon, Korea. ekkim@inha.ac.kr
- 2Department of Environmental Engineering, Anyang University, Anyang, Korea.
- 3Anti-Ageing Research Institute of BIO-FD&C Co., Ltd., Incheon, Korea.
- 4Natuzen Co., Ltd., Incheon, Korea.
- KMID: 2382878
- DOI: http://doi.org/10.5021/ad.2016.28.5.555
Abstract
- BACKGROUND
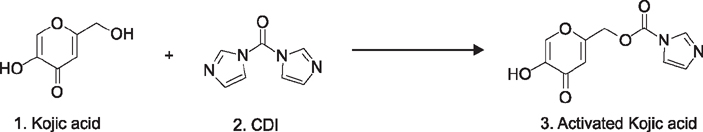
Kojic acid was used for decades in the cosmetic industry as an antimelanogenic agent. However, there are two major drawbacks of Kojic acid, one is cytotoxicity and second are instability on storage. These limitations led the scientist to synthesize the active Kojic acid peptides.
OBJECTIVE
In the present study, we synthesize and investigate the effect of five Kojic acid peptides to overcome the limitation of Kojic acid.
METHODS
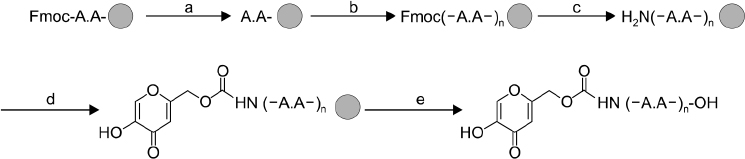
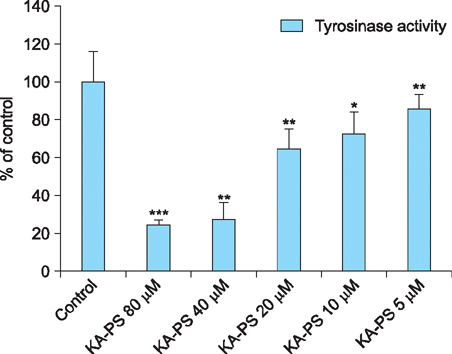
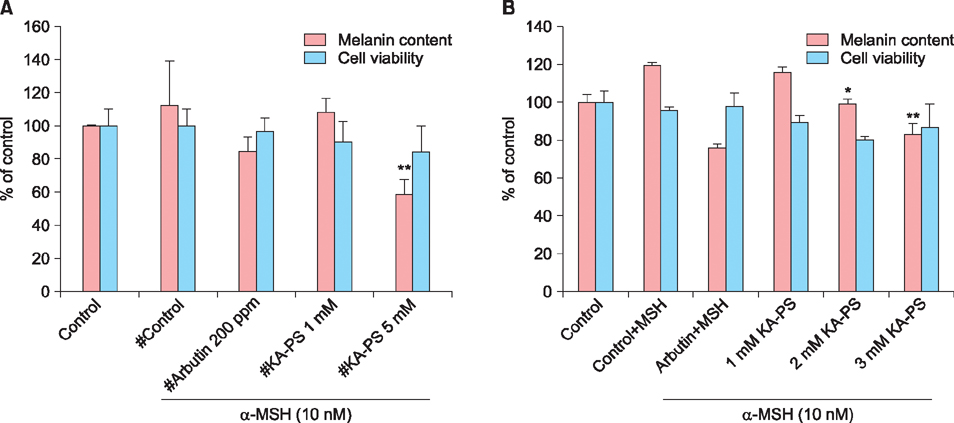
The peptide was analyzed and purified by high-performance liquid chromatography and matrix-assisted laser desorption ionization time of flight mass spectroscopy. Further, the tyrosinase activities of the Kojic acid and Kojic acid peptides were compared. The toxicity was measured and the melanin content is recorded in B16F10 mouse melanoma cells.
RESULTS
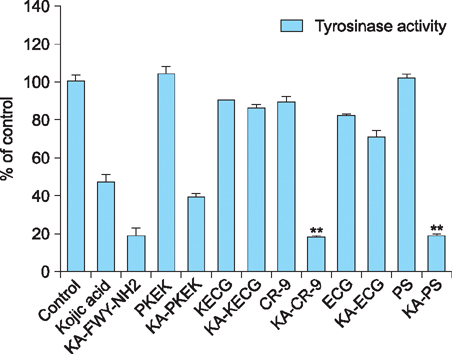
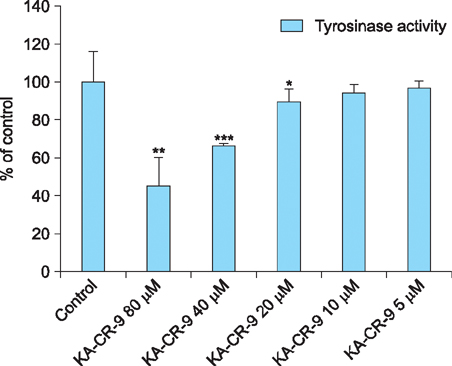
Maximum tyrosinase activity was measured by Kojic acid peptides. Therefore, Kojic acid peptides were subjected to melanin assay and cytotoxicity assay and finally the stability of the Kojic acid peptide was measured.
CONCLUSION
It was observed that this newly synthesized Kojic acid peptide is stable and potent to inhibit the tyrosinase activity and melanin content of B16F10 mouse melanoma cells without exhibiting cell toxicity. Together, these preliminary results suggest that a further exploration is being needed to establish Kojic acid peptide as antimelanogenic agent.
Keyword
MeSH Terms
Figure
Reference
-
1. Jones G. Beauty imagined: a history of the global beauty industry. Oxford and New York: Oxford University Press;2010.2. Noh JM, Kwak SY, Kim DH, Lee YS. Kojic acid-tripeptide amide as a new tyrosinase inhibitor. Biopolymers. 2007; 88:300–307.
Article3. Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004; 84:1155–1228.
Article4. Breathnach AC, Nazzaro-Porro M, Passi S, Zina G. Azelaic acid therapy in disorders of pigmentation. Clin Dermatol. 1989; 7:106–119.
Article5. Verallo-Rowell VM, Verallo V, Graupe K, Lopez-Villafuerte L, Garcia-Lopez M. Double-blind comparison of azelaic acid and hydroquinone in the treatment of melasma. Acta Derm Venereol Suppl (Stockh). 1989; 143:58–61.6. Jimbow K. N-acetyl-4-S-cysteaminylphenol as a new type of depigmenting agent for the melanoderma of patients with melasma. Arch Dermatol. 1991; 127:1528–1534.
Article7. Neering H. Treatment of melasma (chloasma) by local application of a steroid cream. Dermatologica. 1975; 151:349–353.
Article8. Griffiths CE, Finkel LJ, Ditre CM, Hamilton TA, Ellis CN, Voorhees JJ. Topical tretinoin (retinoic acid) improves melasma. A vehicle-controlled, clinical trial. Br J Dermatol. 1993; 129:415–421.
Article9. Kimbrough-Green CK, Griffiths CE, Finkel LJ, Hamilton TA, Bulengo-Ransby SM, Ellis CN, et al. Topical retinoic acid (tretinoin) for melasma in black patients. A vehicle-controlled clinical trial. Arch Dermatol. 1994; 130:727–733.
Article10. Lima LL, Lima RM, da Silva AF, do Carmo AM, da Silva AD, Raposo NR. Azastilbene analogs as tyrosinase inhibitors: new molecules with depigmenting potential. ScientificWorldJournal. 2013; 2013:274643.
Article11. Saghaie L, Pourfarzam M, Fassihi A, Sartippour B. Synthesis and tyrosinase inhibitory properties of some novel derivatives of kojic acid. Res Pharm Sci. 2013; 8:233–242.12. Schurink M, van Berkel WJ, Wichers HJ, Boeriu CG. Novel peptides with tyrosinase inhibitory activity. Peptides. 2007; 28:485–495.
Article13. Chen JS, Wei C, Marshall MR. Inhibition mechanism of kojic acid on polyphenol oxidase. J Agric Food Chem. 1991; 39:1897–1901.
Article14. Kobayashi Y, Kayahara H, Tadasa K, Nakamura T, Tanaka H. Synthesis of amino acid derivatives of kojic acid and their tyrosinase inhibitory activity. Biosci Biotech Biochem. 1995; 59:1745–1746.
Article15. Kadokawa J, Nishikura T, Muraoka R, Tagaya H, Fukuoka N. Synthesis of kojic acid derivatives containing phenolic hydroxy groups. Commun. 2003; 33:1081–1086.
Article16. Kitano T, Tada H, Nishimura T, Teramukai S, Kanai M, Nishimura T, et al. Prevalence and incidence of anemia in Japanese cancer patients receiving outpatient chemotherapy. Int J Hematol. 2007; 86:37–41.
Article17. Manga P, Orlow SJ. Inverse correlation between pink-eyed dilution protein expression and induction of melanogenesis by bafilomycin A1. Pigment Cell Res. 2001; 14:362–367.
Article18. Kubo I, Kinst-Hori I. Tyrosinase inhibitory activity of the olive oil flavor compounds. J Agric Food Chem. 1999; 47:4574–4578.
Article19. Luo LH, Kim HJ, Nguyen DH, Lee HB, Lee NH, Kim EK. Depigmentation of melanocytes by (2Z,8Z)-matricaria acid methyl ester isolated from Erigeron breviscapus. Biol Pharm Bull. 2009; 32:1091–1094.
Article20. Park S, Morya VK, Nguyen DH, Singh BK, Lee HB, Kim EK. Unrevealing the role of P-protein on melanosome biology and structure, using siRNA-mediated down regulation of OCA2. Mol Cell Biochem. 2015; 403:61–71.
Article21. Artursson P, Palm K, Luthman K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv Drug Deliv Rev. 2001; 46:27–43.
Article22. Yoon M, Campbell JL, Andersen ME, Clewell HJ. Quantitative in vitro to in vivo extrapolation of cell-based toxicity assay results. Crit Rev Toxicol. 2012; 42:633–652.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Molecular docking Study of Nuciferine as a Tyrosinase Inhibitor and Its Therapeutic Potential for Hyperpigmentation
- Fungistatic Activity of Kojic Acid Against Human Pathogenic Fungi and Inhibition of Melanin-production in Cryptococcus neoformans
- The Effects of SCH-T2 Seaweed Extract
- Vitis amurensis Ruprecht root inhibited alpha-melanocyte stimulating hormone-induced melanogenesis in B16F10 cells
- Antioxidant, Anti-Melanogenic and Anti-Wrinkle Effects of Phellinus vaninii