J Korean Neurosurg Soc.
2017 May;60(3):355-361. 10.3340/jkns.2016.0505.015.
Effects of Quercetin and Mannitol on Erythropoietin Levels in Rats Following Acute Severe Traumatic Brain Injury
- Affiliations
-
- 1Department of Neurosurgery, School of Medicine and Hospital, Dokuz Eylul University, Izmir, Turkey. okalemci@gmail.com
- 2Department of Pharmacology, Eskisehir Osmangazi University, Eskisehir, Turkey.
- 3Department of Neurosurgery, School of Medicine and Hospital, Dumlupınar University, Kutahya, Turkey.
- 4Department of Neurosurgery, Kilis State Hospital, Kilis, Turkey.
- 5Department of Biostatistics and Medical Informatics, Eskisehir Osmangazi University, Eskisehir, Turkey.
- KMID: 2382771
- DOI: http://doi.org/10.3340/jkns.2016.0505.015
Abstract
OBJECTIVE
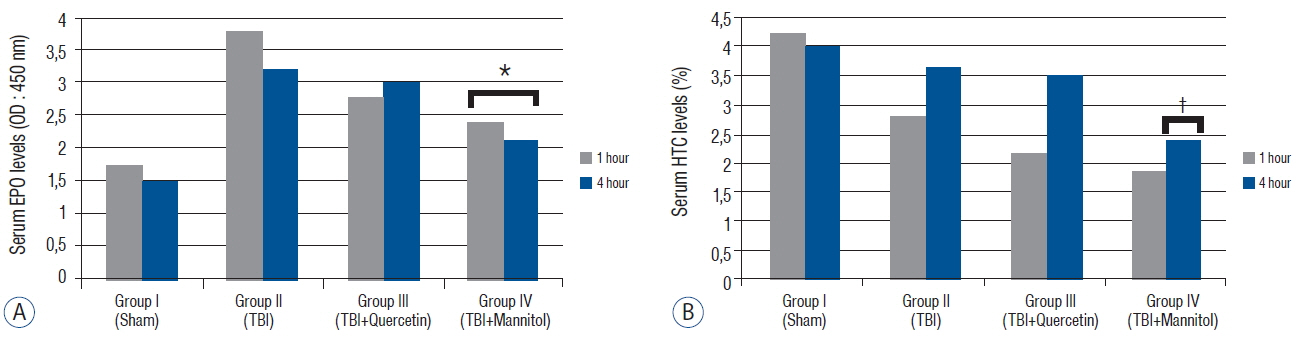
The aim of this study to investigate the normal values of erythropoietin (EPO) and neuroprotective effects of quercetin and mannitol on EPO and hematocrit levels after acute severe traumatic brain injury (TBI) in rat model.
METHODS
A weight-drop impact acceleration model of TBI was used on 40 male Wistar rats. The animals were divided into sham (group I), TBI (group II), TBI+quercetin (50 mg/kg intravenously) (group III), and TBI+mannitol (1 mg/kg intravenously) (group IV) groups. The malondialdehyde, glutathione peroxidase, catalase, EPO, and hematocrit levels were measured 1 and 4 hour after injury. Two-way repeated measures analysis of variance and Tukey's test were used for statistical analysis.
RESULTS
The malondialdehyde levels decreased significantly after administration of quercetin and mannitol compared with those in group II. Catalase and glutathione peroxidase levels increased significantly in groups III and IV. Serum EPO levels decreased significantly after mannitol but not after quercetin administration. Serum hematocrit levels did not change significantly after quercetin and mannitol administration 1 hour after trauma. However, mannitol administration decreased serum hematocrit levels significantly after 4 hour.
CONCLUSION
This study suggests that quercetin may be a good alternative treatment for TBI, as it did not decrease the EPO levels.
Keyword
MeSH Terms
-
Acceleration
Animals
Brain Injuries*
Catalase
Erythropoietin*
Glutathione Peroxidase
Hematocrit
Humans
Male
Malondialdehyde
Mannitol*
Models, Animal
Neuroprotective Agents
Quercetin*
Rats*
Rats, Wistar
Reference Values
Catalase
Erythropoietin
Glutathione Peroxidase
Malondialdehyde
Mannitol
Neuroprotective Agents
Quercetin
Figure
Reference
-
References
1. Akdemir Ozisik P, Oruckaptan H, Ozdemir Geyik P, Misirlioglu M, Sargon MF, Kilinc K, et al. Effect of erythropoietin on brain tissue after experimental head trauma in rats. Surg Neurol. 68:547–555. discussion 555. 2007.
Article2. Ansari MA, Abdul HM, Joshi G, Opii WO, Butterfield DA. Protective effect of quercetin in primary neurons against Abeta(1–42): relevance to Alzheimer’s disease. J Nutr Biochem. 20:269–275. 2009.
Article3. Balak N, Isiksacan N, Turkoglu R. Does serum osmolarity change as a result of the reflex neuroprotective mechanism of cerebral osmo-regulation after minor head trauma? J Korean Neurosurg Soc. 45:151–156. 2009.
Article4. Basarslan SK, Gocmez C, Kamasak K, Ekici MA, Ulutabanca H, Dogu Y, et al. The effects of erythropoietin, dextran and saline on brain edema and lipid peroxidation in experimental head trauma. Ulus Travma Acil Cerrahi Derg. 21:235–240. 2015.5. Bischoff SC. Quercetin: potentials in the prevention and therapy of disease. Curr Opin Clin Nutr Metab Care. 11:733–740. 2008.
Article6. Blaha M, Schwab J, Vajnerova O, Bednar M, Vajner L, Michal T. Intracranial pressure and experimental model of diffuse brain injury in rats. J Korean Neurosurg Soc. 47:7–10. 2010.
Article7. Bleilevens C, Roehl AB, Goetzenich A, Zoremba N, Kipp M, Dang J, et al. Effect of anesthesia and cerebral blood flow on neuronal injury in a rat middle cerebral artery occlusion (MCAO) model. Exp Brain Res. 224:155–164. 2013.
Article8. Bouzat P, Millet A, Boue Y, Pernet-Gallay K, Trouve-Buisson T, Gaide-Chevronnay L, et al. Changes in brain tissue oxygenation after treatment of diffuse traumatic brain injury by erythropoietin. Crit Care Med. 41:1316–1324. 2013.
Article9. Cole TB. Global road safety crisis remedy sought: 1.2 million killed, 50 million injured annually. JAMA. 291:2531–2532. 2004.10. Davis AE. Mechanisms of traumatic brain injury: biomechanical, structural and cellular considerations. Crit Care Nurs Q. 23:1–13. 2000.
Article11. DeWitt DS, Jenkins LW, Prough DS. Enhanced vulnerability to secondary ischemic insults after experimental traumatic brain injury. New Horiz. 3:376–383. 1995.12. Dong YS, Wang JL, Feng DY, Qin HZ, Wen H, Yin ZM, et al. Protective effect of quercetin against oxidative stress and brain edema in an experimental rat model of subarachnoid hemorrhage. Int J Med Sci. 11:282–290. 2014.
Article13. Dugas AJ Jr, Castaneda-Acosta J, Bonin GC, Price KL, Fischer NH, Winston GW. Evaluation of the total peroxyl radical-scavenging capacity of flavonoids: structure-activity relationships. J Nat Prod. 63:327–331. 2000.
Article14. Faria A, Pestana D, Teixeira D, Azevedo J, De Freitas V, Mateus N, et al. Flavonoid transport across RBE4 cells: a blood-brain barrier model. Cell Mol Biol Lett. 15:234–241. 2010.
Article15. Fernandez SP, Wasowski C, Loscalzo LM, Granger RE, Johnston GA, Paladini AC, et al. Central nervous system depressant action of flavonoid glycosides. Eur J Pharmacol. 539:168–176. 2006.
Article16. Ferrali M, Signorini C, Ciccoli L, Bambagioni S, Rossi V, Pompella A, et al. Protection of erythrocytes against oxidative damage and autologous immunoglobulin G (IgG) binding by iron chelator fluor-benzoil-pyridoxal hydrazone. Biochem Pharmacol. 59:1365–1373. 2000.
Article17. Fisher JW. Erythropoietin: physiology and pharmacology update. Exp Biol Med (Maywood). 228:1–14. 2003.
Article18. Ghajar J. Traumatic brain injury. Lancet. 356:923–929. 2000.
Article19. Grasso G, Alafaci C, Buemi M. Erythropoietin in traumatic brain injury: an answer will come soon. World Neurosurg. 84:1491–1492. 2015.
Article20. Grasso G, Sfacteria A, Meli F, Fodale V, Buemi M, Iacopino DG. Neuroprotection by erythropoietin administration after experimental traumatic brain injury. Brain Res. 1182:99–105. 2007.
Article21. Haleagrahara N, Radhakrishnan A, Lee N, Kumar P. Flavonoid quercetin protects against swimming stress-induced changes in oxidative biomarkers in the hypothalamus of rats. Eur J Pharmacol. 621:46–52. 2009.
Article22. Harnly JM, Doherty RF, Beecher GR, Holden JM, Haytowitz DB, Bhagwat S, et al. Flavonoid content of U.S. fruits, vegetables, and nuts. J Agric Food Chem. 54:9966–9977. 2006.
Article23. Hartley CE, Varma M, Fischer JP, Riccardi R, Strauss JA, Shah S, et al. Neuroprotective effects of erythropoietin on acute metabolic and pathological changes in experimentally induced neurotrauma. J Neurosurg. 109:708–714. 2008.
Article24. Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol Ther. 96:67–202. 2002.
Article25. Hellewell SC, Yan EB, Alwis DS, Bye N, Morganti-Kossmann MC. Erythropoietin improves motor and cognitive deficit, axonal pathology, and neuroinflammation in a combined model of diffuse traumatic brain injury and hypoxia, in association with upregulation of the erythropoietin receptor. J Neuroinflammation. 10:156. 2013.
Article26. Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 342:1007–1011. 1993.
Article27. Jager AK, Saaby L. Flavonoids and the CNS. Molecules. 16:1471–1485. 2011.
Article28. Juul SE, Beyer RP, Bammler TK, McPherson RJ, Wilkerson J, Farin FM. Microarray analysis of high-dose recombinant erythropoietin treatment of unilateral brain injury in neonatal mouse hippocampus. Pediatr Res. 65:485–492. 2009.
Article29. Kaufmann AM, Cardoso ER. Aggravation of vasogenic cerebral edema by multiple-dose mannitol. J Neurosurg. 77:584–589. 1992.
Article30. Kook D, Wolf AH, Yu AL, Neubauer AS, Priglinger SG, Kampik A, et al. The protective effect of quercetin against oxidative stress in the human RPE in vitro. Invest Ophthalmol Vis Sci. 49:1712–1720. 2008.
Article31. Lee JY, Lee CY, Kim HR, Lee CH, Kim HW, Kim JH. A role of serum-based neuronal and glial markers as potential predictors for distinguishing severity and related outcomes in traumatic brain injury. J Korean Neurosurg Soc. 58:93–100. 2015.
Article32. Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 7:728–741. 2008.
Article33. Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K. A new model of diffuse brain injury in rats. Part I: Pathophysiology and biomechanics. J Neurosurg. 80:291–300. 1994.34. Middleton E Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 52:673–751. 2000.35. Myhrstad MC, Carlsen H, Nordstrom O, Blomhoff R, Moskaug JO. Flavonoids increase the intracellular glutathione level by transactivation of the gamma-glutamylcysteine synthetase catalytical subunit promoter. Free Radic Biol Med. 32:386–393. 2002.
Article36. Narayan RK, Michel ME, Ansell B, Baethmann A, Biegon A, Bracken MB, et al. Clinical trials in head injury. J Neurotrauma. 19:503–557. 2002.
Article37. Ossola B, Kaariainen TM, Mannisto PT. The multiple faces of quercetin in neuroprotection. Expert Opin Drug Saf. 8:397–409. 2009.
Article38. Park JE, Kim SH, Yoon SH, Cho KG, Kim SH. Risk factors predicting unfavorable neurological outcome during the early period after traumatic brain injury. J Korean Neurosurg Soc. 45:90–95. 2009.
Article39. Peng W, Xing Z, Yang J, Wang Y, Wang W, Huang W. The efficacy of erythropoietin in treating experimental traumatic brain injury: a systematic review of controlled trials in animal models. J Neurosurg. 121:653–664. 2014.
Article40. Rangel-Ordonez L, Noldner M, Schubert-Zsilavecz M, Wurglics M. Plasma levels and distribution of flavonoids in rat brain after single and repeated doses of standardized Ginkgo biloba extract EGb 761®. Planta Med. 76:1683–1690. 2010.
Article41. Robertson CS, Hannay HJ, Yamal JM, Gopinath S, Goodman JC, Tilley BC, et al. Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: a randomized clinical trial. JAMA. 312:36–47. 2014.
Article42. Rossi R, Dalle-Donne I, Milzani A, Giustarini D. Oxidized forms of glutathione in peripheral blood as biomarkers of oxidative stress. Clin Chem. 52:1406–1414. 2006.
Article43. Schober ME, Requena DF, Block B, Davis LJ, Rodesch C, Casper TC, et al. Erythropoietin improved cognitive function and decreased hippocampal caspase activity in rat pups after traumatic brain injury. J Neurotrauma. 31:358–369. 2014.
Article44. Schültke E, Kamencic H, Zhao M, Tian GF, Baker AJ, Griebel RW, et al. Neuroprotection following fluid percussion brain trauma: a pilot study using quercetin. J Neurotrauma. 22:1475–1484. 2005.
Article45. Tango HK, Schmidt AP, Mizumoto N, Lacava M, Cruz RJ Jr, Auler JO Jr. Low hematocrit levels increase intracranial pressure in an animal model of cryogenic brain injury. J Trauma. 66:720–726. 2009.
Article46. Thurman D, Guerrero J. Trends in hospitalization associated with traumatic brain injury. JAMA. 282:954–957. 1999.
Article47. Velly L, Pellegrini L, Guillet B, Bruder N, Pisano P. Erythropoietin 2nd cerebral protection after acute injuries: a double-edged sword? Pharmacol Ther. 128:445–459. 2010.
Article48. Yang T, Kong B, Gu JW, Kuang YQ, Cheng L, Yang WT, et al. Anti-apoptotic and anti-oxidative roles of quercetin after traumatic brain injury. Cell Mol Neurobiol. 34:797–804. 2014.
Article49. Yilmaz N, Dulger H, Kiymaz N, Yilmaz C, Gudu BO, Demir I. Activity of mannitol and hypertonic saline therapy on the oxidant and antioxidant system during the acute term after traumatic brain injury in the rats. Brain Res. 1164:132–135. 2007.
Article50. Youdim KA, Dobbie MS, Kuhnle G, Proteggente AR, Abbott NJ, Rice-Evans C. Interaction between flavonoids and the blood-brain barrier: in vitro studies. J Neurochem. 85:180–192. 2003.
Article51. Zhang Y, Xiong Y, Mahmood A, Meng Y, Qu C, Schallert T, et al. Therapeutic effects of erythropoietin on histological and functional outcomes following traumatic brain injury in rats are independent of hematocrit. Brain Res. 1294:153–64. 2009.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Hypertonic Saline and Mannitol against Edema Formation after Cryogenic Brain Injury in Rats
- High glucose stimulates the expression of erythropoietin in rat glomerular epithelial cells
- Mannitol induced acute oliguric renal failure
- Controversies in Acute Care of Patients with Severe Traumatic Brain Injury
- Is the Phenomenon of Blood-Brain Barrier Disruption Induced with Mannitol All-or-None?