Lab Anim Res.
2011 Sep;27(3):245-250. 10.5625/lar.2011.27.3.245.
High glucose stimulates the expression of erythropoietin in rat glomerular epithelial cells
- Affiliations
-
- 1Bio-therapy Human Resources Center, Animal Medical Center and Department of Veterinary Physiology, College of Veterinary Medicine, Chonnam National University, Gwangju, Korea. parksh@chonnam.ac.kr
- KMID: 1850052
- DOI: http://doi.org/10.5625/lar.2011.27.3.245
Abstract
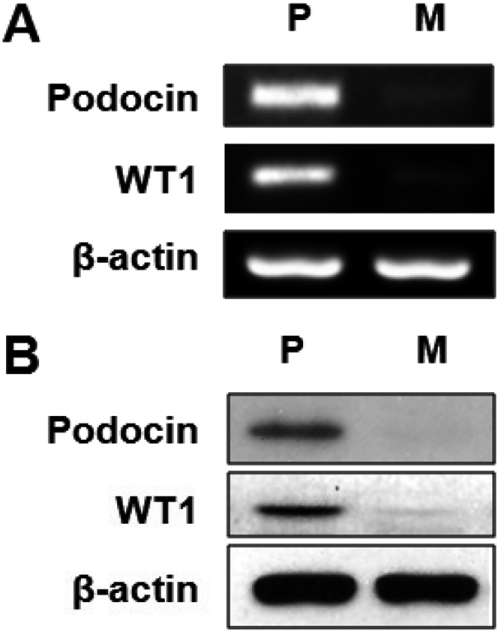
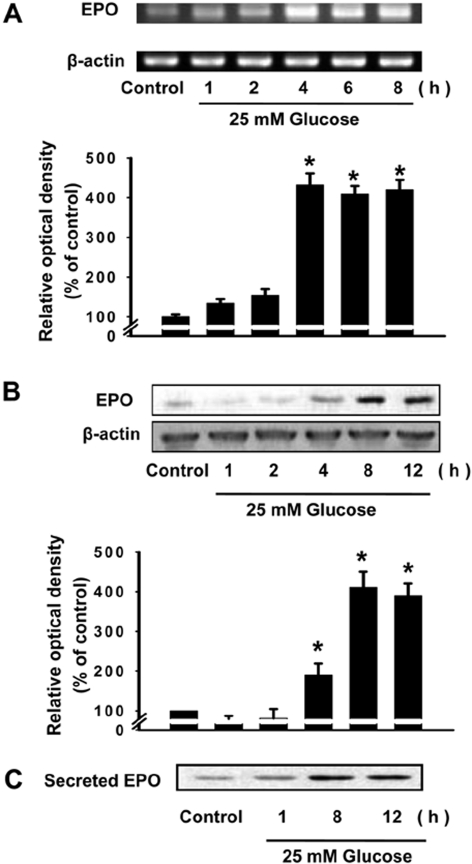
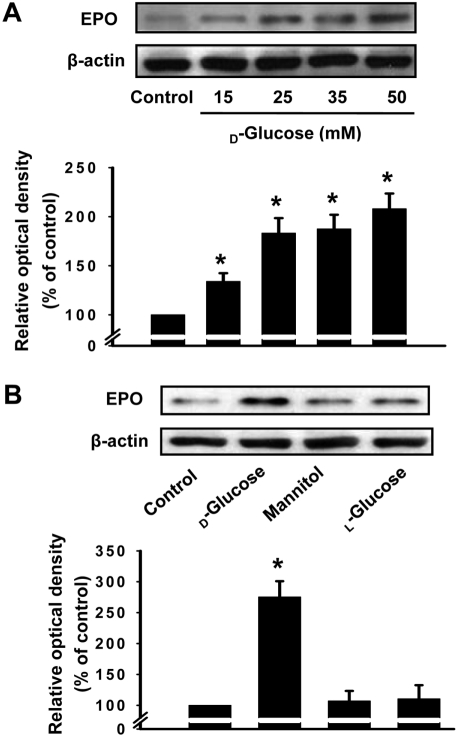
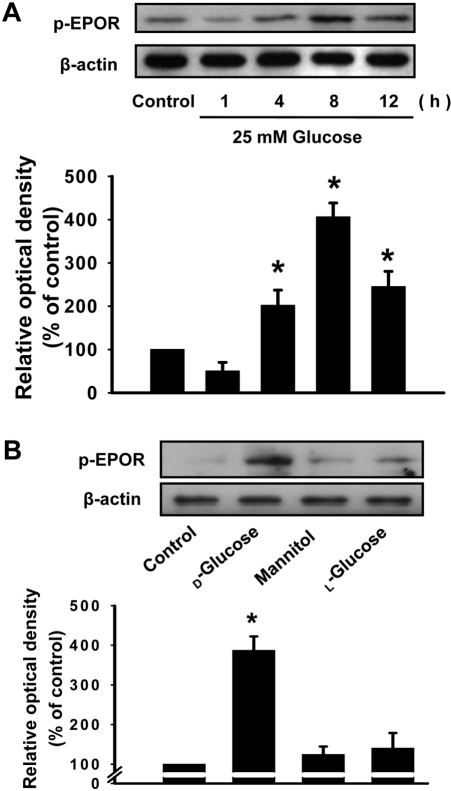
- It has been reported that the levels of erythropoietin are associated with diabetes mellitus. Glomerular epithelial cells, located in the renal cortex, play an important role in the regulation of kidney function and hyperglycemia-induced cell loss of glomerular epithelial cells is implicated in the onset of diabetic nephropathy. This study investigated the effect of high glucose on erythropoietin and erythropoietin receptor expression in rat glomerular epithelial cells. We found that 25 mM D-glucose, but not mannitol or L-glucose, stimulated erythropoietin mRNA and protein expression in a time dependent manner (>4 h) in rat glomerular epithelial cells. In addition, 25 mM glucose, but not mannitol or L-glucose, also increased the phosphorylation of erythropoietin receptor, suggesting a role for erythropoietin receptor phosphorylation in erythropoietin synthesis. We conclude that high glucose stimulates erythropoietin production and erythropoietin receptor phosphorylation in rat glomerular epithelial cells.
MeSH Terms
Figure
Reference
-
1. Dronavalli S, Duka I, Bakris GL. The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab. 2008; 4:444–452. PMID: 18607402.
Article2. Menini S, Iacobini C, Oddi G, Ricci C, Simonelli P, Fallucca S, Grattarola M, Pugliese F, Pesce C, Pugliese G. Increased glomerular cell (podocyte) apoptosis in rats with streptozotocin-induced diabetes mellitus: role in the development of diabetic glomerular disease. Diabetologia. 2007; 50:2591–2599. PMID: 17901943.
Article3. Fisher JW, Koury S, Ducey T, Mendel S. Erythropoietin production by interstitial cells of hypoxic monkey kidneys. Br J Haematol. 1996; 95:27–32. PMID: 8857934.
Article4. Jelkmann W, Metzen E. Erythropoietin in the control of red cell production. Ann Anat. 1996; 178:391–403. PMID: 8931850.
Article5. Dunlop EA, Percy MJ, Boland MP, Maxwell AP, Lappin TR. Induction of signalling in non-erythroid cells by pharmacological levels of erythropoietin. Neurodegener Dis. 2006; 3:94–100. PMID: 16909043.
Article6. Cariou A, Andre S, Claessens YE. Extra-hematopoietic effects of erythropoietin. Cardiovasc Hematol Disord Drug Targets. 2008; 8:173–178. PMID: 18781929.
Article7. Choi D, Retnakaran R, Woo M. The extra-hematopoietic role of erythropoietin in diabetes mellitus. Curr Diabetes Rev. 2011; 7:284–290. PMID: 21644916.
Article8. Wagner M, Alam A, Zimmermann J, Rauh K, Koljaja-Batzner A, Raff U, Wanner C, Schramm L. Endogenous erythropoietin and the association with inflammation and mortality in diabetic chronic kidney disease. Clin J Am Soc Nephrol. 2011; 6:1573–1579. PMID: 21734083.
Article9. Kreisberg JI, Hoover RL, Karnovsky MJ. Isolation and characterization of rat glomerular epithelial cells in vitro. Kidney Int. 1978; 14:21–30. PMID: 682422.10. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72:248–254. PMID: 942051.
Article11. Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, Johnson RS, Simon MC, Keith B, Haase VH. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest. 2007; 117:1068–1077. PMID: 17404621.12. Kim I, Kim CH, Yim YS, Ahn YS. Autocrine function of erythropoietin in IGF-1-induced erythropoietin biosynthesis. Neuroreport. 2008; 19:1699–1703. PMID: 18841090.
Article13. Mori S, Saito T, Morishita Y, Saito K, Urabe A, Wakabayashi T, Takaku F. Glomerular epithelium as the main locus of erythropoietin in human kidney. Jpn J Exp Med. 1985; 55:69–70. PMID: 4046211.14. Loya F, Yang Y, Lin H, Goldwasser E, Albitar M. Transgenic mice carrying the erythropoietin gene promoter linked to lacZ express the reporter in proximal convoluted tubule cells after hypoxia. Blood. 1994; 84:1831–1836. PMID: 8080988.
Article15. Mojiminiyi OA, Abdella NA, Zaki MY, El Gebely SA, Mohamedi HM, Aldhahi WA. Prevalence and associations of low plasma erythropoietin in patients with Type 2 diabetes mellitus. Diabet Med. 2006; 23:839–844. PMID: 16911620.
Article16. Symeonidis A, Kouraklis-Symeonidis A, Psiroyiannis A, Leotsinidis M, Kyriazopoulou V, Vassilakos P, Vagenakis A, Zoumbos N. Inappropriately low erythropoietin response for the degree of anemia in patients with noninsulin-dependent diabetes mellitus. Ann Hematol. 2006; 85:79–85. PMID: 16132904.
Article17. Choi D, Schroer SA, Lu SY, Wang L, Wu X, Liu Y, Zhang Y, Gaisano HY, Wagner KU, Wu H, Retnakaran R, Woo M. Erythropoietin protects against diabetes through direct effects on pancreatic beta cells. J Exp Med. 2010; 207:2831–2842. PMID: 21149549.18. Dang J, Jia R, Tu Y, Xiao S, Ding G. Erythropoietin prevents reactive oxygen species generation and renal tubular cell apoptosis at high glucose level. Biomed Pharmacother. 2010; 64(10):681–685. PMID: 20685070.
Article19. Teramo KA, Hiilesmaa VK, Schwartz R, Clemons GK, Widness JA. Amniotic fluid and cord plasma erythropoietin levels in pregnancies complicated by preeclampsia, pregnancy-induced hypertension and chronic hypertension. J Perinat Med. 2004; 32:240–247. PMID: 15188798.
Article20. Echigoya MH, Obikane K, Nakashima T, Sasaki S. Glomerular localization of erythropoietin receptor mRNA and protein in neonatal and mature mouse kidney. Nephron Exp Nephrol. 2005; 100:e21–e29. PMID: 15741742.
Article21. Satoh K, Kagaya Y, Nakano M, Ito Y, Ohta J, Tada H, Karibe A, Minegishi N, Suzuki N, Yamamoto M, Ono M, Watanabe J, Shirato K, Ishii N, Sugamura K, Shimokawa H. Important role of endogenous erythropoietin system in recruitment of endothelial progenitor cells in hypoxia-induced pulmonary hypertension in mice. Circulation. 2006; 113:1442–1450. PMID: 16534010.
Article22. Asaumi Y, Kagaya Y, Takeda M, Yamaguchi N, Tada H, Ito K, Ohta J, Shiroto T, Shirato K, Minegishi N, Shimokawa H. Protective role of endogenous erythropoietin system in nonhematopoietic cells against pressure overload-induced left ventricular dysfunction in mice. Circulation. 2007; 115:2022–2032. PMID: 17404160.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of High Glucose and TGF-beta on the Cellular Injury in Cultured Glomerular Epithelial Cells
- Colocalization of ANG II and mRNA for the Renin-Angiotensin System Components in Cultured Rat Glomerular Epithelial and Mesangial Cells
- High Glucose and Advanced Glycosylation Endproducts (AGE) Modulate the CD2AP Expression in Glomerular Epithelial Cells (GEpC)
- Effects of High Glucose and Advanced Glycosylation Endproducts (AGE) on the alpha-actinin-4 Expressed by Glomerular Epithelial Cells (GEpC)
- High Glucose and Advanced Glycosylation Endproducts(AGE) Modulate the P-cadherin Expression in Glomerular Epithelial Cells(GEpC)