Anesth Pain Med.
2017 Apr;12(2):117-122. 10.17085/apm.2017.12.2.117.
Predictive performance of target controlled infusion of propofol-MCT/LCT using the modified Marsh and Schnider models: a simulation study
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. byungmoonchoi7@gmail.com
- 2Department of Clinical Pharmacology and Therapeutics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2379730
- DOI: http://doi.org/10.17085/apm.2017.12.2.117
Abstract
- BACKGROUND
Only two pharmacokinetic models of propofol are commercially available in the category of target controlled infusion (TCI) pumps: the modified Marsh and Schnider models. Both models were developed using propofol-LCT (long chain triglyceride). Depending on the excipient, the pharmacokinetic properties of fast-acting drugs, such as propofol, vary. Hence, it is necessary to evaluate the predictive performances of both models using propofol-MCT (medium chain triglyceride)/LCT, which is frequently used in clinical practice.
METHODS
This was a computer simulation study, using data collected in the previous clinical analysis used to evaluate the predictive performance of a pharmacokinetic model of propofol-MCT/LCT. The infusion profiles for each patient were applied as inputs to both models. Simulations were performed using TCI software, and the simulated plasma concentrations of both models were calculated.
RESULTS
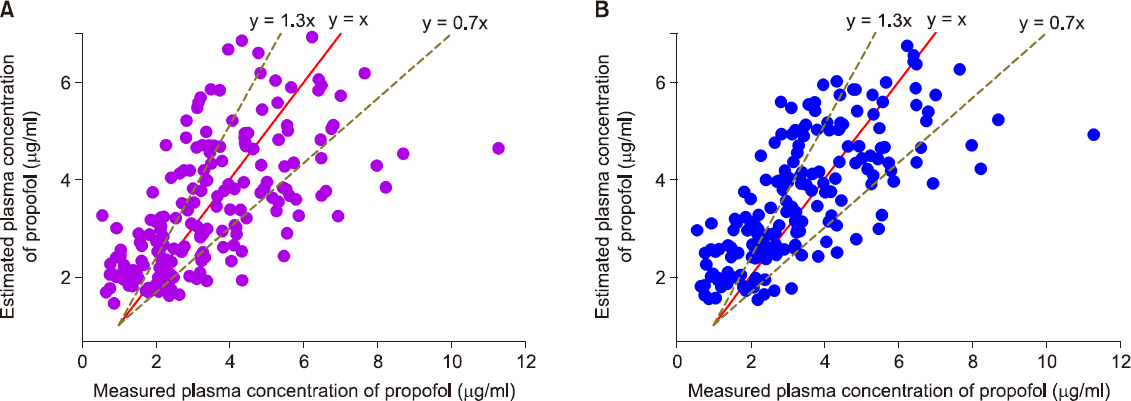
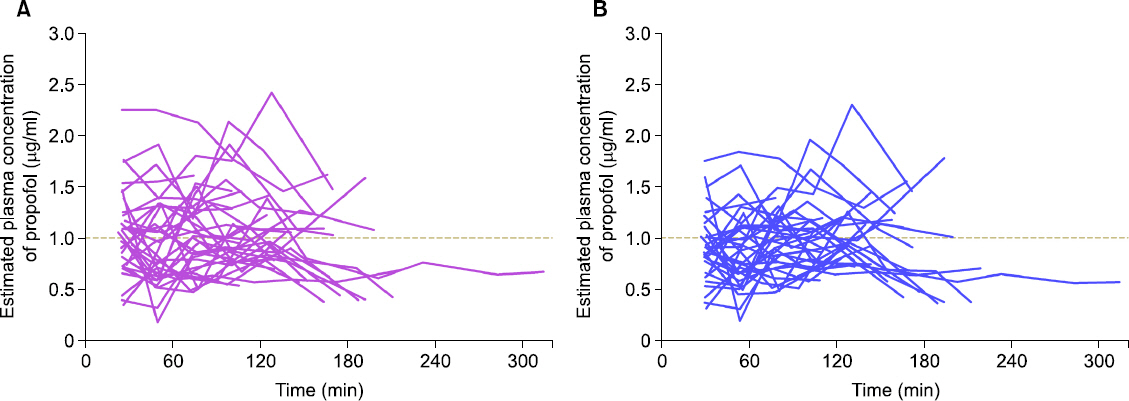
In total, 217 plasma samples, obtained from 35 patients, were used to determine the predictive performance. The pooled median (95% CI) biases and inaccuracies were 9.6 (−1.7 to 15.4) and 32.1 (22.6-38.2) respectively, for the modified Marsh model, and −5.9 (−8.9 to −0.7) and 26.3 (21.7-27.8) respectively, for the Schnider model.
CONCLUSIONS
Although the pooled bias and inaccuracy of the Schnider models were clinically acceptable (< 10-20% and approximately 20-30%, respectively), the Schnider model consistently produced negatively biased predictions. Conversely, even though the pooled inaccuracy of the modified Marsh model failed to meet this criterion, the value did not deviate significantly from the standard. Therefore, it is reasonable to conclude that both TCI models can be used for propofol-MCT/LCT.
Keyword
MeSH Terms
Figure
Reference
-
1. van den Nieuwenhuyzen MC, Engbers FH, Vuyk J, Burm AG. Target-controlled infusion systems:role in anaesthesia and analgesia. Clin Pharmacokinet. 2000; 38:181–90. DOI: 10.2165/00003088-200038020-00003. PMID: 10709777.2. Absalom AR, Glen JI, Zwart GJ, Schnider TW, Struys MM. Target-controlled infusion:a mature technology. Anesth Analg. 2016; 122:70–8. DOI: 10.1213/ANE.0000000000001009. PMID: 26516798.3. Marsh B, White M, Morton N, Kenny GN. Pharmacokinetic model driven infusion of propofol in children. Br J Anaesth. 1991; 67:41–8. DOI: 10.1093/bja/67.1.41. PMID: 1859758.4. Struys MM, De Smet T, Depoorter B, Versichelen LF, Mortier EP, Dumortier FJ, et al. Comparison of plasma compartment versus two methods for effect compartment--controlled target-controlled infusion for propofol. Anesthesiology. 2000; 92:399–406. DOI: 10.1097/00000542-200002000-00021. PMID: 10691226.5. Schnider TW, Minto CF, Gambus PL, Andresen C, Goodale DB, Shafer SL, et al. The influence of method of administration and covariates on the pharmacokinetics of propofol in adult volunteers. Anesthesiology. 1998; 88:1170–82. DOI: 10.1097/00000542-199805000-00006. PMID: 9605675.6. Baker MT, Naguib M. Propofol:the challenges of formulation. Anesthesiology. 2005; 103:860–76. DOI: 10.1097/00000542-200510000-00026.7. Lee EH, Lee SH, Park DY, Ki KH, Lee EK, Lee DH, et al. Physicochemical properties, pharmacokinetics, and pharmacodynamics of a reformulated microemulsion propofol in rats. Anesthesiology. 2008; 109:436–47. DOI: 10.1097/ALN.0b013e318182a486. PMID: 18719441.8. Allford MA, Mensah JA. Discomfort on injection:a comparison between two formulations of propofol. Eur J Anaesthesiol. 2006; 23:971–4. DOI: 10.1017/S0265021506001049. PMID: 16824242.9. Kim KM, Choi BM, Park SW, Lee SH, Christensen LV, Zhou J, et al. Pharmacokinetics and pharmacodynamics of propofol microemulsion and lipid emulsion after an intravenous bolus and variable rate infusion. Anesthesiology. 2007; 106:924–34. DOI: 10.1097/01.anes.0000265151.78943.af. PMID: 17457123.10. Cox EH, Knibbe CA, Koster VS, Langemeijer MW, Tukker EE, Lange R, et al. Influence of different fat emulsion-based intravenous formulations on the pharmacokinetics and pharmacodynamics of propofol. Pharm Res. 1998; 15:442–8. DOI: 10.1023/A:1011980432646. PMID: 9563075.11. Ward DS, Norton JR, Guivarc’h PH, Litman RS, Bailey PL. Pharmacodynamics and pharmacokinetics of propofol in a medium-chain triglyceride emulsion. Anesthesiology. 2002; 97:1401–8. DOI: 10.1097/00000542-200212000-00011. PMID: 12459665.12. Glen JB, Servin F. Evaluation of the predictive performance of four pharmacokinetic models for propofol. Br J Anaesth. 2009; 102:626–32. DOI: 10.1093/bja/aep043. PMID: 19297371.13. Beal SL, Sheiner LB, Boeckmann A, Bauer RJ. NONMEM users guides. NONMEM Project Group, University of California. San Francisco;1992.14. Glen JB, White M. A comparison of the predictive performance of three pharmacokinetic models for propofol using measured values obtained during target-controlled infusion. Anaesthesia. 2014; 69:550–7. DOI: 10.1111/anae.12631. PMID: 24720380.15. Varvel JR, Donoho DL, Shafer SL. Measuring the predictive performance of computer-controlled infusion pumps. J Pharmacokinet Biopharm. 1992; 20:63–94. DOI: 10.1007/BF01143186. PMID: 1588504.16. Rau J, Roizen MF, Doenicke AW, O’Connor MF, Strohschneider U. Propofol in an emulsion of long- and medium-chain triglycerides:the effect on pain. Anesth Analg. 2001; 93:382–4. DOI: 10.1097/00000539-200108000-00029. PMID: 11473865.17. Leisure GS, O’Flaherty J, Green L, Jones DR. Propofol and postoperative pancreatitis. Anesthesiology. 1996; 84:224–7. DOI: 10.1097/00000542-199601000-00027. PMID: 8572338.18. Wicklmayr M, Rett K, Dietze G, Mehnert H. Comparison of metabolic clearance rates of MCT/LCT and LCT emulsions in diabetics. JPEN J Parenter Enteral Nutr. 1988; 12:68–71. DOI: 10.1177/014860718801200168. PMID: 3125359.19. Choi BM, Lee HG, Byon HJ, Lee SH, Lee EK, Kim HS, et al. Population pharmacokinetic and pharmacodynamic model of propofol externally validated in children. J Pharmacokinet Pharmacodyn. 2015; 42:163–77. DOI: 10.1007/s10928-015-9408-2. PMID: 25724290.20. Choi BM, Lee HG, Byon HJ, Lee SH, Lee EK, Kim HS, et al. Algorithms to rapidly achieve and maintain stable drug concentrations at the site of drug effect with a computer-controlled infusion pump. J Pharmacokinet Biopharm. 1992; 20:147–69. DOI: 10.1007/BF01070999.21. Absalom AR, Lee M, Menon DK, Sharar SR, De Smet T, Halliday J, et al. Predictive performance of the Domino, Hijazi, and Clements models during low-dose target-controlled ketamine infusions in healthy volunteers. Br J Anaesth. 2007; 98:615–23. DOI: 10.1093/bja/aem063. PMID: 17389691. PMCID: PMC3838936.22. Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean bodyweight. Clin Pharmacokinet. 2005; 44:1051–65. DOI: 10.2165/00003088-200544100-00004. PMID: 16176118.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of the clinical performance of the modified Marsh model for propofol between underweight and normal-weight patients with Crohn's disease
- Effect-site target-controlled infusion of propofol: comparison of Schnider and modified Marsh models
- Cross-simulation between two pharmacokinetic models for the target-controlled infusion of propofol
- Pain Intensity at Injection Site during Esophagogastroduodenoscopy Using Long- and MediumChain versus Long-Chain Triglyceride Propofol: A Randomized Controlled Double-Blind Study
- Evaluation of Marsh's Pharmacokinetic Parameter Set for Target Controlled Infusion of Propofol in Korean