Ewha Med J.
2017 Apr;40(2):77-86. 10.12771/emj.2017.40.2.77.
Role of βâ‚-Integrin in Colorectal Cancer: Case-Control Study
- Affiliations
-
- 1Department of Surgery, Ewha Womans University School of Medicine, Seoul, Korea. ralee@ewha.ac.kr
- KMID: 2379502
- DOI: http://doi.org/10.12771/emj.2017.40.2.77
Abstract
OBJECTIVES
In the metastatic process, interactions between circulating tumor cells (CTCs) and the extracellular matrix or surrounding cells are required. β1-integrin may mediate these interactions. The aim of this study was to investigate whether β1-integrin is associated with the detection of CTCs in colorectal cancer.
METHODS
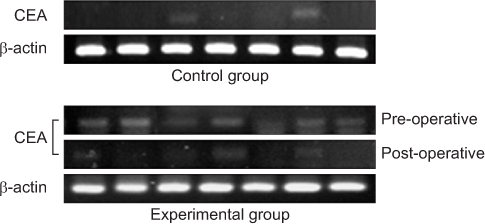
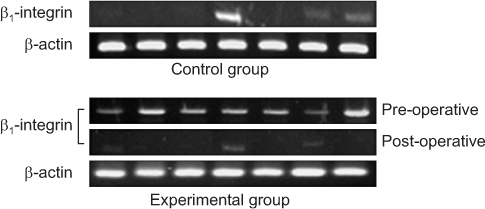
We enrolled 30 patients with colorectal cancer (experimental group) and 30 patients with benign diseases (control group). Blood samples were obtained from each group, carcinoembryonic antigen (CEA) mRNA for CTCs marker and β1-integrin mRNA levels were estimated by using reverse transcription-polymerase chain reaction, and the results were compared between the two groups.
RESULTS
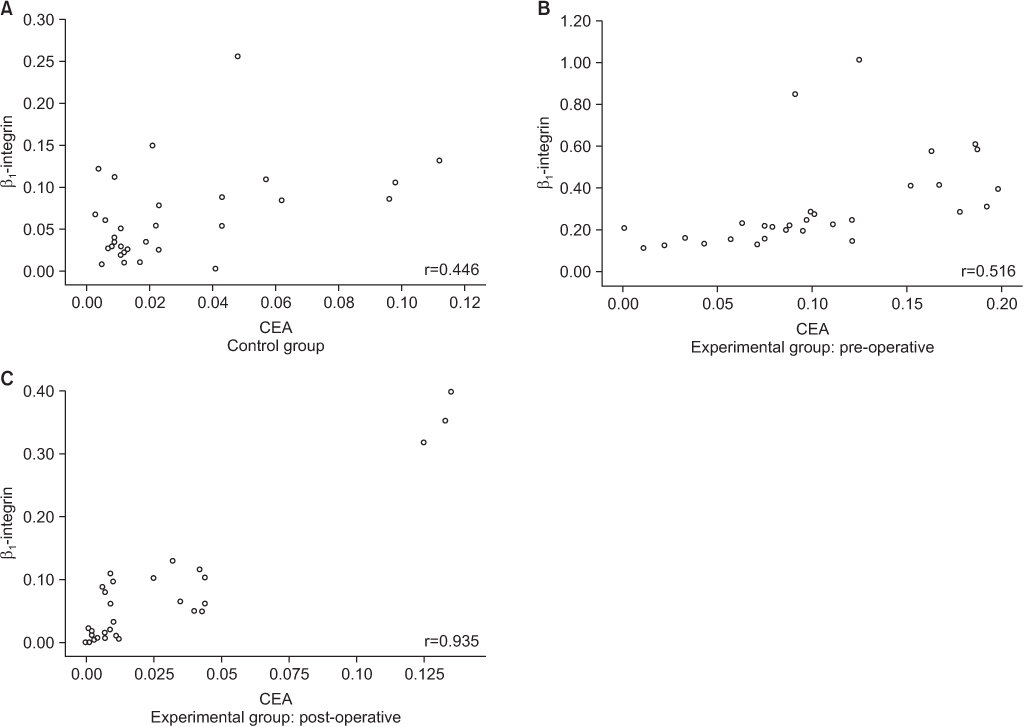
CEA mRNA was detected more frequently in colorectal cancer patients than in control patients (P=0.008). CEA mRNA was significantly reduced after surgery in the colorectal cancer patients (P=0.032). β1-integrin mRNA was detected more in colorectal cancer patients than in the patients with benign diseases (P<0.001). In colorectal cancer patients, expression of β1-integrin mRNA was detected more for advanced-stage cancer than for early-stage cancer (P=0.033) and was significantly decreased after surgery (P<0.001). In addition, expression of β1-integrin mRNA was significantly associated with that of CEA mRNA in colorectal cancer patients (P=0.001).
CONCLUSION
In conclusion, β1-integrin is a potential prognostic factor following surgical resection in colorectal cancer patients. β1-integrin may be a candidate for use as a marker for early detection of micrometastatic tumor cells and for monitoring the therapeutic response in colorectal cancer patients.
MeSH Terms
Figure
Reference
-
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.2. Steinert G, Scholch S, Koch M, Weitz J. Biology and significance of circulating and disseminated tumour cells in colorectal cancer. Langenbecks Arch Surg. 2012; 397:535–542.3. de Castro-Carpeno J, Belda-Iniesta C, Casado Saenz E, Hernandez Agudo E, Feliu Batlle J, Gonzalez Baron M. EGFR and colon cancer: a clinical view. Clin Transl Oncol. 2008; 10:6–13.4. Liu Y, Qian J, Feng JG, Ju HX, Zhu YP, Feng HY, et al. Detection of circulating tumor cells in peripheral blood of colorectal cancer patients without distant organ metastases. Cell Oncol (Dordr). 2013; 36:43–53.5. Barbazan J, Alonso-Alconada L, Muinelo-Romay L, Vieito M, Abalo A, Alonso-Nocelo M, et al. Molecular characterization of circulating tumor cells in human metastatic colorectal cancer. PLoS One. 2012; 7:e40476.6. Thorsteinsson M, Jess P. The clinical significance of circulating tumor cells in non-metastatic colorectal cancer: a review. Eur J Surg Oncol. 2011; 37:459–465.7. Alunni-Fabbroni M, Sandri MT. Circulating tumour cells in clinical practice: mthods of detection and possible characterization. Methods. 2010; 50:289–297.8. Haier J, Nasralla M, Nicolson GL. Cell surface molecules and their prognostic values in assessing colorectal carcinomas. Ann Surg. 2000; 231:11–24.9. Jinka R, Kapoor R, Sistla PG, Raj TA, Pande G. Alterations in cell-extracellular matrix interactions during progression of cancers. Int J Cell Biol. 2012; 2012:219196.10. Wang JY, Wu CH, Lu CY, Hsieh JS, Wu DC, Huang SY, et al. Molecular detection of circulating tumor cells in the peripheral blood of patients with colorectal cancer using RT-PCR: significance of the prediction of postoperative metastasis. World J Surg. 2006; 30:1007–1013.11. Bolocan A, Ion D, Ciocan DN, Paduraru DN. Prognostic and predictive factors in colorectal cancer. Chirurgia (Bucur). 2012; 107:555–563.12. Jiang WQ, Fu FF, Li YX, Wang WB, Wang HH, Jiang HP, et al. Molecular biomarkers of colorectal cancer: prognostic and predictive tools for clinical practice. J Zhejiang Univ Sci B. 2012; 13:663–675.13. Ashworth TR. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust Med J. 1869; 14:146–149.14. Raeisossadati R, Farshchian M, Ganji A, Tavassoli A, Velayati A, Dadkhah E, et al. Quantitative analysis of TEM-8 and CEA tumor markers indicating free tumor cells in the peripheral blood of colorectal cancer patients. Int J Colorectal Dis. 2011; 26:1265–1270.15. Sastre J, Maestro ML, Puente J, Veganzones S, Alfonso R, Rafael S, et al. Circulating tumor cells in colorectal cancer: correlation with clinical and pathological variables. Ann Oncol. 2008; 19:935–938.16. Lu CY, Uen YH, Tsai HL, Chuang SC, Hou MF, Wu DC, et al. Molecular detection of persistent postoperative circulating tumour cells in stages II and III colon cancer patients via multiple blood sampling: prognostic significance of detection for early relapse. Br J Cancer. 2011; 104:1178–1184.17. Aggarwal C, Meropol NJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, et al. Relationship among circulating tumor cells, CEA and overall survival in patients with metastatic colorectal cancer. Ann Oncol. 2013; 24:420–428.18. Wong SC, Chan CM, Ma BB, Hui EP, Ng SS, Lai PB, et al. Clinical significance of cytokeratin 20-positive circulating tumor cells detected by a refined immunomagnetic enrichment assay in colorectal cancer patients. Clin Cancer Res. 2009; 15:1005–1012.19. Smirnov DA, Zweitzig DR, Foulk BW, Miller MC, Doyle GV, Pienta KJ, et al. Global gene expression profiling of circulating tumor cells. Cancer Res. 2005; 65:4993–4997.20. Sato N, Hayashi N, Imamura Y, Tanaka Y, Kinoshita K, Kurashige J, et al. Usefulness of transcription-reverse transcription concerted reaction method for detecting circulating tumor cells in patients with colorectal cancer. Ann Surg Oncol. 2012; 19:2060–2065.21. Wai Wong C, Dye DE, Coombe DR. The role of immunoglobulin superfamily cell adhesion molecules in cancer metastasis. Int J Cell Biol. 2012; 2012:340296.22. Muller V, Riethdorf S, Rack B, Janni W, Fasching PA, Solomayer E, et al. Prospective evaluation of serum tissue inhibitor of metalloproteinase 1 and carbonic anhydrase IX in correlation to circulating tumor cells in patients with metastatic breast cancer. Breast Cancer Res. 2011; 13:R71.23. Zieglschmid V, Hollmann C, Mannel J, Albert W, Jaeschke-Melli S, Eckstein B, et al. Tumor-associated gene expression in disseminated tumor cells correlates with disease progression and tumor stage in colorectal cancer. Anticancer Res. 2007; 27:1823–1832.24. Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010; 10:9–22.25. Rathinam R, Alahari SK. Important role of integrins in the cancer biology. Cancer Metastasis Rev. 2010; 29:223–237.26. Millard M, Odde S, Neamati N. Integrin targeted therapeutics. Theranostics. 2011; 1:154–188.27. Yu MK, Park J, Jeong YY, Moon WK, Jon S. Integrin-targeting thermally cross-linked superparamagnetic iron oxide nanoparticles for combined cancer imaging and drug delivery. Nanotechnology. 2010; 21:415102.28. Park CC, Zhang H, Pallavicini M, Gray JW, Baehner F, Park CJ, et al. Beta1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res. 2006; 66:1526–1535.29. Stoeltzing O, Liu W, Reinmuth N, Fan F, Parry GC, Parikh AA, et al. Inhibition of integrin alpha5beta1 function with a small peptide (ATN-161) plus continuous 5-FU infusion reduces colorectal liver metastases and improves survival in mice. Int J Cancer. 2003; 104:496–503.30. Nam JM, Chung Y, Hsu HC, Park CC. beta1 integrin targeting to enhance radiation therapy. Int J Radiat Biol. 2009; 85:923–928.31. Huang WS, Chen CN, Sze CI, Teng CC. Visfatin induces stromal cell-derived factor-1 expression by β1 integrin signaling in colorectal cancer cells. J Cell Physiol. 2013; 228:1017–1024.32. Paschos KA, Canovas D, Bird NC. The role of cell adhesion molecules in the progression of colorectal cancer and the development of liver metastasis. Cell Signal. 2009; 21:665–674.33. Hsu RY, Chan CH, Spicer JD, Rousseau MC, Giannias B, Rousseau S, et al. LPS-induced TLR4 signaling in human colorectal cancer cells increases beta1 integrin-mediated cell adhesion and liver metastasis. Cancer Res. 2011; 71:1989–1998.34. Andrews EJ, Wang JH, Winter DC, Laug WE, Redmond HP. Tumor cell adhesion to endothelial cells is increased by endotoxin via an upregulation of beta-1 integrin expression. J Surg Res. 2001; 97:14–19.35. Carbonell WS, DeLay M, Jahangiri A, Park CC, Aghi MK. β1 integrin targeting potentiates antiangiogenic therapy and inhibits the growth of bevacizumab-resistant glioblastoma. Cancer Res. 2013; 73:3145–3154.36. Yang B, Gao J, Rao Z, Shen Q. Clinicopathological and prognostic significance of α5β1-integrin and MMP-14 expressions in colorectal cancer. Neoplasma. 2013; 60:254–261.37. Bartolome RA, Barderas R, Torres S, Fernandez-Acenero MJ, Mendes M, Garcia-Foncillas J, et al. Cadherin-17 interacts with α2β1 integrin to regulate cell proliferation and adhesion in colorectal cancer cells causing liver metastasis. Oncogene. 2014; 33:1658–1669.38. Wong CS, Cheung MT, Ma BB, Pun Hui E, Chan AC, Chan CK, et al. Isolated tumor cells and circulating CK20 mRNA in pN0 colorectal cancer patients. Int J Surg Pathol. 2008; 16:119–126.39. Koyanagi K, Bilchik AJ, Saha S, Turner RR, Wiese D, McCarter M, et al. Prognostic relevance of occult nodal micrometastases and circulating tumor cells in colorectal cancer in a prospective multicenter trial. Clin Cancer Res. 2008; 14:7391–7396.40. Ohlsson L, Hammarstrom ML, Israelsson A, Naslund L, Oberg A, Lindmark G, et al. Biomarker selection for detection of occult tumour cells in lymph nodes of colorectal cancer patients using real-time quantitative RT-PCR. Br J Cancer. 2006; 95:218–225.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of beta1-Integrin in Colorectal Cancer: Case-Control Study
- Increased Expression of Thymosin β₄ Is Independently Correlated with Hypoxia Inducible Factor-1α (HIF-1α) and Worse Clinical Outcome in Human Colorectal Cancer
- A Case-Control Study on the Relationship between Obesity and Female Colorectal Cancer
- The role of microbiome in colorectal carcinogenesis and its clinical potential as a target for cancer treatment
- Invasiveness of and Drug Sensitivity to Various Anti-cancer Regimens in Five Colorectal Cancer Cell Lines