Korean J Physiol Pharmacol.
2017 May;21(3):327-334. 10.4196/kjpp.2017.21.3.327.
Dust particles-induced intracellular Ca²⺠signaling and reactive oxygen species in lung fibroblast cell line MRC5
- Affiliations
-
- 1Department of Physiology, College of Medicine, Gachon University, Lee Gil Ya Cancer and Diabetes Institute, Incheon 21999, Korea. minicleo@gachon.ac.kr
- 2Department of Oral Biology, BK21 PLUS Project, Yonsei University College of Dentistry, Seoul 03722, Korea.
- 3Division of Pulmonary, Allergy and Critical Care Medicine, Gachon University, Gil Medical Center, Incheon 21565, Korea.
- KMID: 2376961
- DOI: http://doi.org/10.4196/kjpp.2017.21.3.327
Abstract
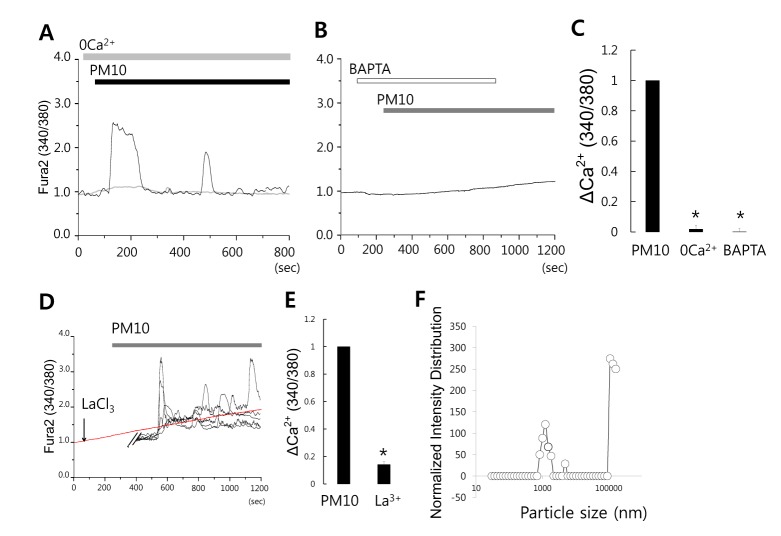
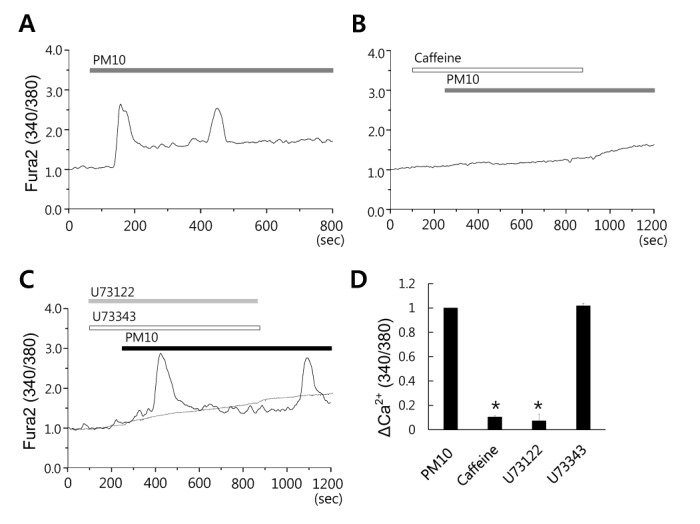
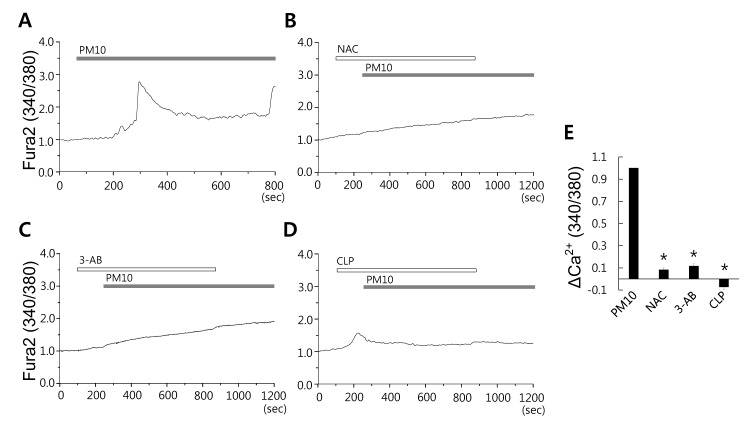
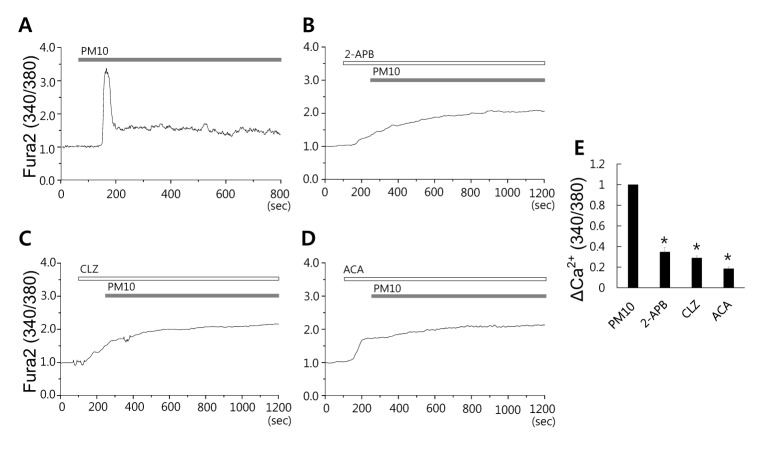
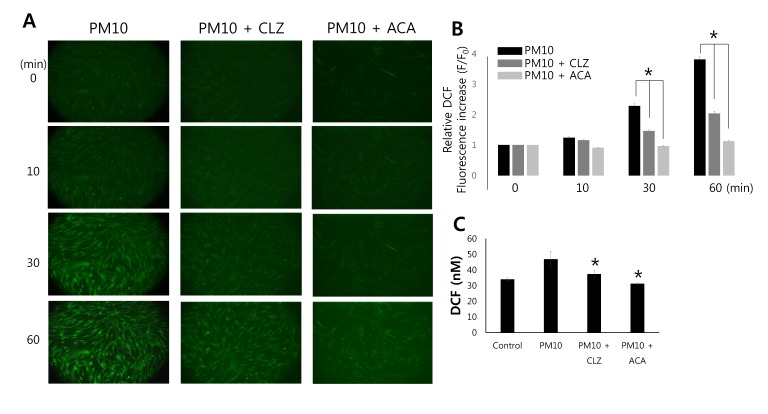
- Epidemiologic interest in particulate matter (PM) is growing particularly because of its impact of respiratory health. It has been elucidated that PM evoked inflammatory signal in pulmonary epithelia. However, it has not been established Ca²âº signaling mechanisms involved in acute PM-derived signaling in pulmonary fibroblasts. In the present study, we explored dust particles PM modulated intracellular Ca²âº signaling and sought to provide a therapeutic strategy by antagonizing PM-induced intracellular Ca²âº signaling in human lung fibroblasts MRC5 cells. We demonstrated that PM10, less than 10 µm, induced intracellular Ca²âº signaling, which was mediated by extracellular Ca²âº. The PM10-mediated intracellular Ca²âº signaling was attenuated by antioxidants, phospholipase blockers, polyADPR polymerase 1 inhibitor, and transient receptor potential melastatin 2 (TRPM2) inhibitors. In addition, PM-mediated increases in reactive oxygen species were attenuated by TRPM2 blockers, clotrimazole (CLZ) and N-(p-amylcinnamoyl) anthranilic acid (ACA). Our results showed that PM10 enhanced reactive oxygen species signal by measuring DCF fluorescence and the DCF signal attenuated by both TRPM2 blockers CLZ and ACA. Here, we suggest functional inhibition of TRPM2 channels as a potential therapeutic strategy for modulation of dust particle-mediated signaling and oxidative stress accompanying lung diseases.
Keyword
MeSH Terms
Figure
Reference
-
1. Kanatani KT, Ito I, Al-Delaimy WK, Adachi Y, Mathews WC, Ramsdell JW. Toyama Asian Desert Dust and Asthma Study Team. Desert dust exposure is associated with increased risk of asthma hospitalization in children. Am J Respir Crit Care Med. 2010; 182:1475–1481. PMID: 20656941.
Article2. He M, Ichinose T, Yoshida S, Yamamoto S, Inoue K, Takano H, Yanagisawa R, Nishikawa M, Mori I, Sun G, Shibamoto T. Asian sand dust enhances murine lung inflammation caused by Klebsiella pneumoniae. Toxicol Appl Pharmacol. 2012; 258:237–247. PMID: 22118940.
Article3. Yeo NK, Hwang YJ, Kim ST, Kwon HJ, Jang YJ. Asian sand dust enhances rhinovirus-induced cytokine secretion and viral replication in human nasal epithelial cells. Inhal Toxicol. 2010; 22:1038–1045. PMID: 20879958.
Article4. Wilker EH, Preis SR, Beiser AS, Wolf PA, Au R, Kloog I, Li W, Schwartz J, Koutrakis P, DeCarli C, Seshadri S, Mittleman MA. Long-term exposure to fine particulate matter, residential proximity to major roads and measures of brain structure. Stroke. 2015; 46:1161–1166. PMID: 25908455.
Article5. Shah AS, Lee KK, McAllister DA, Hunter A, Nair H, Whiteley W, Langrish JP, Newby DE, Mills NL. Short term exposure to air pollution and stroke: systematic review and meta-analysis. BMJ. 2015; 350:h1295. PMID: 25810496.
Article6. Blank F, Stumbles PA, Seydoux E, Holt PG, Fink A, Rothen-Rutishauser B, Strickland DH, von Garnier C. Size-dependent uptake of particles by pulmonary antigen-presenting cell populations and trafficking to regional lymph nodes. Am J Respir Cell Mol Biol. 2013; 49:67–77. PMID: 23492193.
Article7. Tsuda A, Rogers RA, Hydon PE, Butler JP. Chaotic mixing deep in the lung. Proc Natl Acad Sci U S A. 2002; 99:10173–10178. PMID: 12119385.
Article8. Oberdörster G. Pulmonary effects of inhaled ultrafine particles. Int Arch Occup Environ Health. 2001; 74:1–8. PMID: 11196075.9. Philipson K, Falk R, Svartengren M, Jarvis N, Bailey M, Bergmann R, Hofmann W, Camner P. Does lung retention of inhaled particles depend on their geometric diameter? Exp Lung Res. 2000; 26:437–455. PMID: 11033767.10. Choi HS, Ashitate Y, Lee JH, Kim SH, Matsui A, Insin N, Bawendi MG, Semmler-Behnke M, Frangioni JV, Tsuda A. Rapid translocation of nanoparticles from the lung airspaces to the body. Nat Biotechnol. 2010; 28:1300–1303. PMID: 21057497.
Article11. Mohammad AK, Amayreh LK, Mazzara JM, Reineke JJ. Rapid lymph accumulation of polystyrene nanoparticles following pulmonary administration. Pharm Res. 2013; 30:424–434. PMID: 22992832.
Article12. Ichinose T, Yoshida S, Hiyoshi K, Sadakane K, Takano H, Nishikawa M, Mori I, Yanagisawa R, Kawazato H, Yasuda A, Shibamoto T. The effects of microbial materials adhered to Asian sand dust on allergic lung inflammation. Arch Environ Contam Toxicol. 2008; 55:348–357. PMID: 18227959.
Article13. Kyung SY, Yoon JY, Kim YJ, Lee SP, Park JW, Jeong SH. Asian dust particles induce TGF-β(1) via reactive oxygen species in bronchial epithelial cells. Tuberc Respir Dis (Seoul). 2012; 73:84–92. PMID: 23166540.14. Honda A, Matsuda Y, Murayama R, Tsuji K, Nishikawa M, Koike E, Yoshida S, Ichinose T, Takano H. Effects of Asian sand dust particles on the respiratory and immune system. J Appl Toxicol. 2014; 34:250–257. PMID: 23576315.
Article15. Kim ST, Ye MK, Shin SH. Effects of Asian sand dust on mucin gene expression and activation of nasal polyp epithelial cells. Am J Rhinol Allergy. 2011; 25:303–306. PMID: 22186242.
Article16. Shin SH, Ye MK, Hwang YJ, Kim ST. The effect of Asian sand dust-activated respiratory epithelial cells on activation and migration of eosinophils. Inhal Toxicol. 2013; 25:633–639. PMID: 24044679.
Article17. Cohen MD, Vaughan JM, Garrett B, Prophete C, Horton L, Sisco M, Kodavanti UP, Ward WO, Peltier RE, Zelikoff J, Chen LC. Acute high-level exposure to WTC particles alters expression of genes associated with oxidative stress and immune function in the lung. J Immunotoxicol. 2015; 12:140–153. PMID: 24911330.
Article18. Higashisaka K, Fujimura M, Taira M, Yoshida T, Tsunoda S, Baba T, Yamaguchi N, Nabeshi H, Yoshikawa T, Nasu M, Yoshioka Y, Tsutsumi Y. Asian dust particles induce macrophage inflammatory responses via mitogen-activated protein kinase activation and reactive oxygen species production. J Immunol Res. 2014; 2014:856154. DOI: 10.1155/2014/856154. PMID: 24987712.
Article19. Sakamoto N, Hayashi S, Gosselink J, Ishii H, Ishimatsu Y, Mukae H, Hogg JC, van Eeden SF. Calcium dependent and independent cytokine synthesis by air pollution particle-exposed human bronchial epithelial cells. Toxicol Appl Pharmacol. 2007; 225:134–141. PMID: 17720209.
Article20. Lansley AB, Sanderson MJ, Dirksen ER. Control of the beat cycle of respiratory tract cilia by Ca2+ and cAMP. Am J Physiol. 1992; 263:L232–L242. PMID: 1325130.21. Hayashi T, Kawakami M, Sasaki S, Katsumata T, Mori H, Yoshida H, Nakahari T. ATP regulation of ciliary beat frequency in rat tracheal and distal airway epithelium. Exp Physiol. 2005; 90:535–544. PMID: 15769883.
Article22. Abdullah LH, Conway JD, Cohn JA, Davis CW. Protein kinase C and Ca2+ activation of mucin secretion in airway goblet cells. Am J Physiol. 1997; 273:L201–L210. PMID: 9252557.23. Davis CW. Regulation of mucin secretion from in vitro cellular models. Novartis Found Symp. 2002; 248:113–125. discussion 125-131, 277-282. PMID: 12568491.
Article24. Sakamoto Y, Ishijima M, Kaneko H, Kurebayashi N, Ichikawa N, Futami I, Kurosawa H, Arikawa-Hirasawa E. Distinct mechanosensitive Ca2+ influx mechanisms in human primary synovial fibroblasts. J Orthop Res. 2010; 28:859–864. PMID: 20108315.25. Murata N, Ito S, Furuya K, Takahara N, Naruse K, Aso H, Kondo M, Sokabe M, Hasegawa Y. Ca2+ influx and ATP release mediated by mechanical stretch in human lung fibroblasts. Biochem Biophys Res Commun. 2014; 453:101–105. PMID: 25256743.26. Wakui M, Osipchuk YV, Petersen OH. Receptor-activated cytoplasmic Ca2+ spiking mediated by inositol trisphosphate is due to Ca2+-induced Ca2+ release. Cell. 1990; 63:1025–1032. PMID: 1701691.27. Ehrlich BE, Kaftan E, Bezprozvannaya S, Bezprozvanny I. The pharmacology of intracellular Ca2+-release channels. Trends Pharmacol Sci. 1994; 15:145–149. PMID: 7754532.28. Criddle DN, Sutton R, Petersen OH. Role of Ca2+ in pancreatic cell death induced by alcohol metabolites. J Gastroenterol Hepatol. 2006; 21(Suppl 3):S14–S17. PMID: 16958662.29. Wiegman CH, Michaeloudes C, Haji G, Narang P, Clarke CJ, Russell KE, Bao W, Pavlidis S, Barnes PJ, Kanerva J, Bittner A, Rao N, Murphy MP, Kirkham PA, Chung KF, Adcock IM. COPDMAP. Oxidative stress-induced mitochondrial dysfunction drives inflammation and airway smooth muscle remodeling in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2015; 136:769–780. PMID: 25828268.
Article30. Park L, Wang G, Moore J, Girouard H, Zhou P, Anrather J, Iadecola C. The key role of transient receptor potential melastatin-2 channels in amyloid-β-induced neurovascular dysfunction. Nat Commun. 2014; 5:5318. PMID: 25351853.
Article31. Shekh K, Khan S, Jena G, Kansara BR, Kushwaha S. 3-Aminobenzamide--a PARP inhibitor enhances the sensitivity of peripheral blood micronucleus and comet assays in mice. Toxicol Mech Methods. 2014; 24:332–341. PMID: 24568202.32. Choi SY, Kim YH, Lee YK, Kim KT. Chlorpromazine inhibits store-operated calcium entry and subsequent noradrenaline secretion in PC12 cells. Br J Pharmacol. 2001; 132:411–418. PMID: 11159689.
Article33. Mori Y, Kajimoto T, Nakao A, Takahashi N, Kiyonaka S. Receptor signaling integration by TRP channelsomes. Adv Exp Med Biol. 2011; 704:373–389. PMID: 21290307.
Article34. Takahashi N, Kozai D, Kobayashi R, Ebert M, Mori Y. Roles of TRPM2 in oxidative stress. Cell Calcium. 2011; 50:279–287. PMID: 21616534.
Article35. Nazıroğlu M, Özgül C, Çelik Ö, Çiğ B, Sözbir E. Aminoethoxydiphenyl borate and flufenamic acid inhibit Ca2+ influx through TRPM2 channels in rat dorsal root ganglion neurons activated by ADP-ribose and rotenone. J Membr Biol. 2011; 241:69–75. PMID: 21509529.36. Roberge S, Roussel J, Andersson DC, Meli AC, Vidal B, Blandel F, Lanner JT, Le Guennec JY, Katz A, Westerblad H, Lacampagne A, Fauconnier J. TNF-α-mediated caspase-8 activation induces ROS production and TRPM2 activation in adult ventricular myocytes. Cardiovasc Res. 2014; 103:90–99. PMID: 24802330.
Article37. Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003; 4:517–529. PMID: 12838335.
Article38. Lei B, Hitomi H, Mori T, Nagai Y, Deguchi K, Mori H, Masaki T, Nakano D, Kobori H, Kitaura Y, Nishiyama A. Effect of efonidipine on TGF-β1-induced cardiac fibrosis through Smad2-dependent pathway in rat cardiac fibroblasts. J Pharmacol Sci. 2011; 117:98–105. PMID: 21897055.
Article39. Ohyama T, Sato K, Kishimoto K, Yamazaki Y, Horiguchi N, Ichikawa T, Kakizaki S, Takagi H, Izumi T, Mori M. Azelnidipine is a calcium blocker that attenuates liver fibrosis and may increase antioxidant defence. Br J Pharmacol. 2012; 165:1173–1187. PMID: 21790536.
Article40. Mishima K, Maeshima A, Miya M, Sakurai N, Ikeuchi H, Hiromura K, Nojima Y. Involvement of N-type Ca2+ channels in the fibrotic process of the kidney in rats. Am J Physiol Renal Physiol. 2013; 304:F665–F673. PMID: 23324177.41. Xu R, Li Q, Zhou XD, Perelman JM, Kolosov VP. Oxidative stress mediates the disruption of airway epithelial tight junctions through a TRPM2-PLCγ1-PKCα signaling pathway. Int J Mol Sci. 2013; 14:9475–9486. PMID: 23629676.42. Horng T. Calcium signaling and mitochondrial destabilization in the triggering of the NLRP3 inflammasome. Trends Immunol. 2014; 35:253–261. PMID: 24646829.
Article43. Li R, Kou X, Geng H, Xie J, Yang Z, Zhang Y, Cai Z, Dong C. Effect of ambient PM(2.5) on lung mitochondrial damage and fusion/fission gene expression in rats. Chem Res Toxicol. 2015; 28:408–418. PMID: 25560372.
Article44. Dellinger B, Pryor WA, Cueto R, Squadrito GL, Hegde V, Deutsch WA. Role of free radicals in the toxicity of airborne fine particulate matter. Chem Res Toxicol. 2001; 14:1371–1377. PMID: 11599928.
Article45. Kinnula VL, Fattman CL, Tan RJ, Oury TD. Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy. Am J Respir Crit Care Med. 2005; 172:417–422. PMID: 15894605.46. Park JW, Yoon JY, Kim YJ, Kyung SY, Lee SP, Jeong SH, Moon C. Extracellular signal-regulated kinase (ERK) inhibition attenuates cigarette smoke extract (CSE) induced-death inducing signaling complex (DISC) formation in human lung fibroblasts (MRC-5) cells. J Toxicol Sci. 2010; 35:33–39. PMID: 20118622.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hydrogen peroxide attenuates refilling of intracellular calcium store in mouse pancreatic acinar cells
- Hydrogen peroxide inhibits Ca²⺠efflux through plasma membrane Ca²âº-ATPase in mouse parotid acinar cells
- Role of Reactive Oxygen Species in Cell Death Pathways
- Asian Dust Particles Induce TGF-beta1 via Reactive Oxygen Species in Bronchial Epithelial Cells
- Effect of Erythromycin on Pro-inflammatory Signalings by Particles