Investig Magn Reson Imaging.
2017 Mar;21(1):1-8. 10.13104/imri.2017.21.1.1.
Dual Component Analysis for In Vivo T₂* Decay of Hyperpolarized ¹³C Metabolites
- Affiliations
-
- 1Department of Electrical and Electronic Engineering, Yonsei University, Seoul, Korea. donghyunkim@yonsei.ac.kr
- 2Center for Neuroscience Imaging Research, Institute for Basic Science, Sungkyunkwan University, Suwon, Korea.
- 3Department of Radiology, College of Medicine, Yonsei University, Seoul, Korea.
- KMID: 2376263
- DOI: http://doi.org/10.13104/imri.2017.21.1.1
Abstract
- PURPOSE
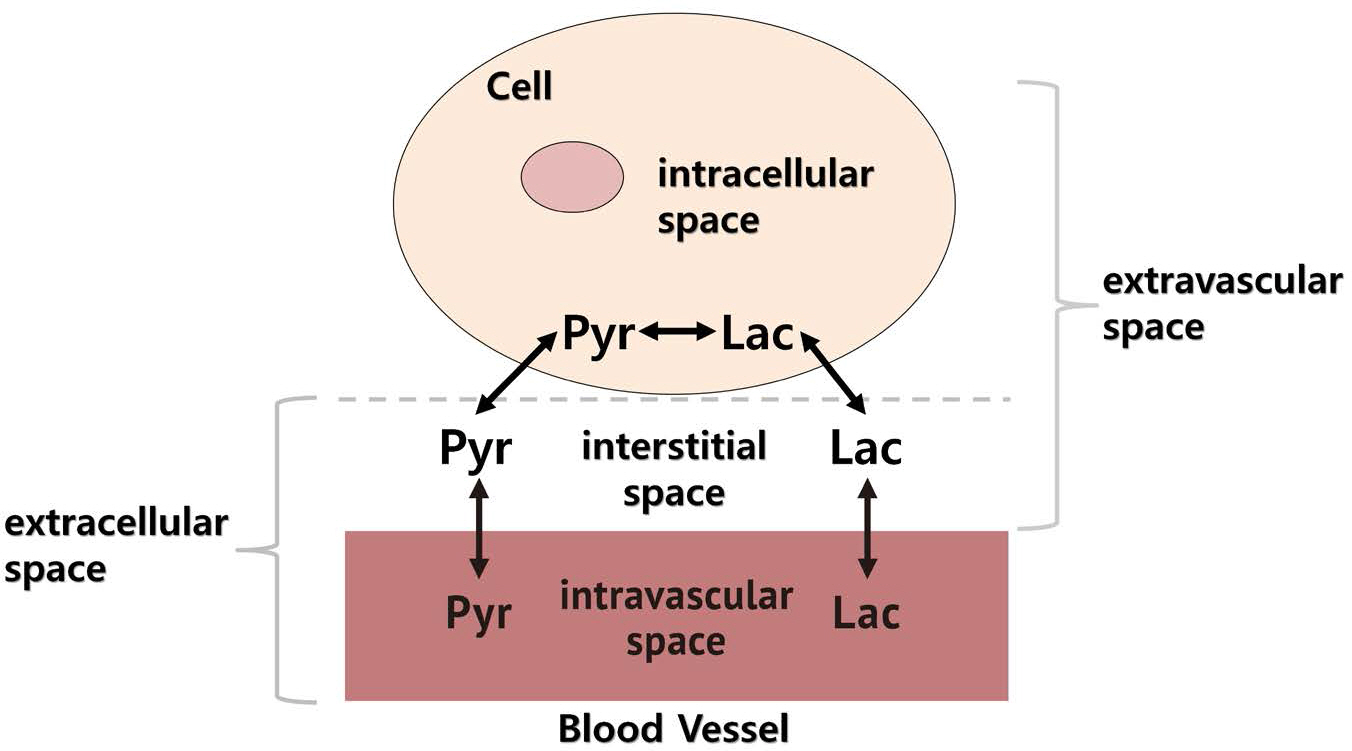
To investigate the exchange and redistribution of hyperpolarized ¹³C metabolites between different pools by temporally analyzing the relative fraction of dual Tâ‚‚* components of hyperpolarized ¹³C metabolites.
MATERIALS AND METHODS
A dual exponential decay analysis of Tâ‚‚* is performed for [1-¹³C] pyruvate and [1-¹³C] lactate using nonspatially resolved dynamic ¹³C MR spectroscopy from mice brains with tumors (n = 3) and without (n = 4) tumors. The values of shorter and longer Tâ‚‚* components are explored when fitted from averaged spectrum and temporal variations of their fractions.
RESULTS
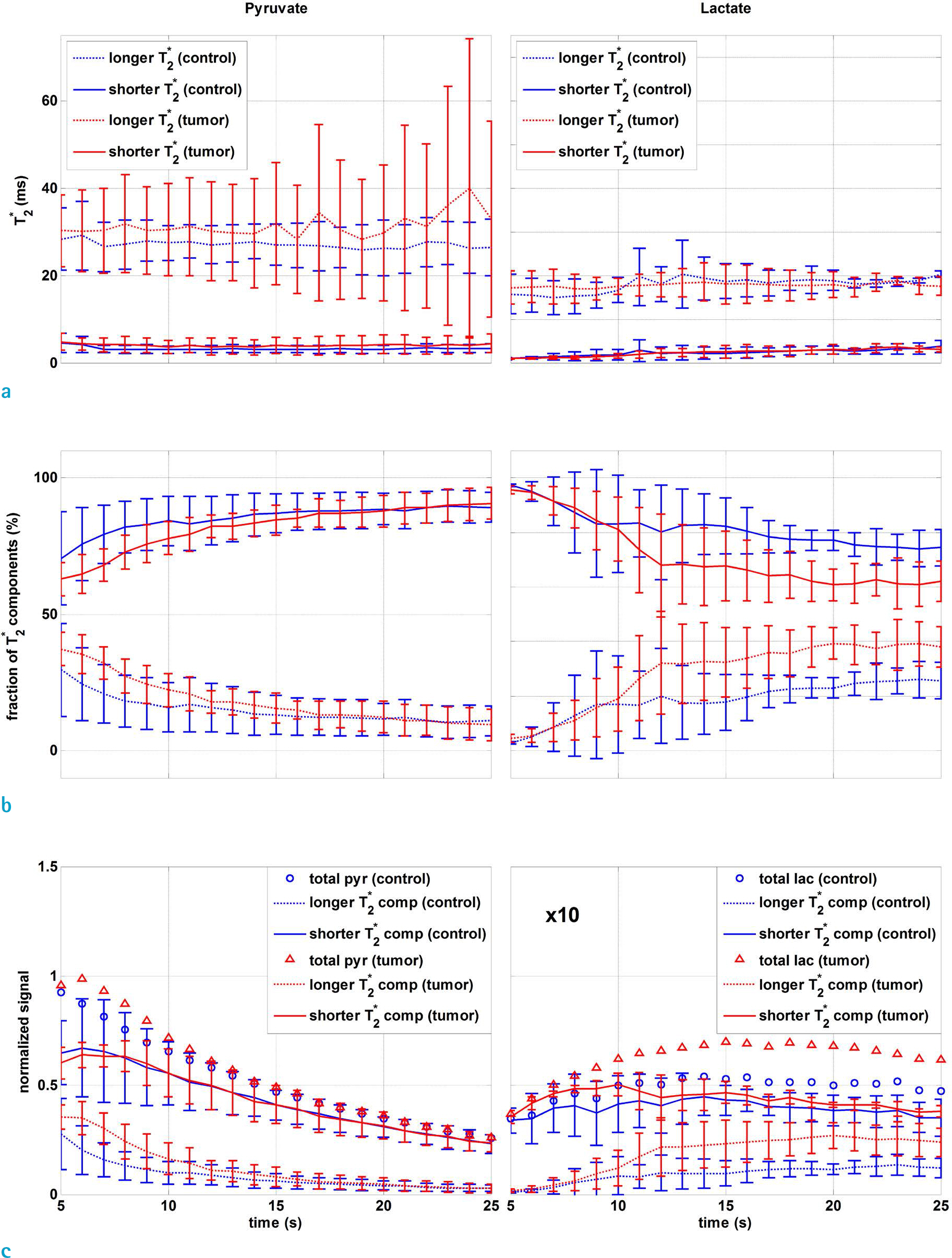
The Tâ‚‚* values were not significantly different between the tumor and control groups, but the fraction of longer Tâ‚‚* [1-¹³C] lactate components was more than 10% in the tumor group over that of the controls (P < 0.1). The fraction of shorter Tâ‚‚* components of [1-¹³C] pyruvate showed an increasing tendency while that of the [1-¹³C] lactate was decreasing over time. The slopes of the changing fraction were steeper for the tumor group than the controls, especially for lactate (P < 0.01). In both pyruvate and lactate, the fraction of the shorter Tâ‚‚* component was always greater than the longer Tâ‚‚* component over time.
CONCLUSIONS
The exchange and redistribution of pyruvate and lactate between different pools was investigated by dual component analysis of the free induction decay signal from hyperpolarized ¹³C experiments. Tumor and control groups showed differences in their fractions rather than the values of longer and shorter Tâ‚‚* components. Fraction changing dynamics may provide an aspect for extravasation and membrane transport of pyruvate and lactate, and will be useful to determine the appropriate time window for acquisition of hyperpolarized ¹³C images.
Keyword
MeSH Terms
Figure
Reference
-
1. Ardenkjaer-Larsen JH, Fridlund B, Gram A, et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci U S A. 2003; 100:10158–10163.2. Kurhanewicz J, Vigneron DB, Brindle K, et al. Analysis of cancer metabolism by imaging hyperpolarized nuclei: prospects for translation to clinical research. Neoplasia. 2011; 13:81–97.
Article3. Day SE, Kettunen MI, Gallagher FA, et al. Detecting tumor response to treatment using hyperpolarized 13C magnetic resonance imaging and spectroscopy. Nat Med. 2007; 13:1382–1387.
Article4. Warburg O. On the origin of cancer cells. Science. 1956; 123:309–314.
Article5. Kettunen MI, Kennedy BW, Hu DE, Brindle KM. Spin echo measurements of the extravasation and tumor cell uptake of hyperpolarized [1-(13) C]lactate and [1-(13) C]pyruvate. Magn Reson Med. 2013; 70:1200–1209.6. Romijn JA, Chinkes DL, Schwarz JM, Wolfe RR. Lactate-pyruvate interconversion in blood: implications for in vivo tracer studies. Am J Physiol. 1994; 266:E334–340.
Article7. Yen YF, Le Roux PR, Bok R, et al. Apparent T2 of 13C-labeled metabolites in vivo. In. Proceedings of the 16th Annual Meeting of ISMRM. Toronto: Canada;2008. p. 1747.8. Kettunen MI, Hu DE, Witney TH, et al. Magnetization transfer measurements of exchange between hyperpolarized [1-13C]pyruvate and [1-13C]lactate in a murine lymphoma. Magn Reson Med. 2010; 63:872–880.
Article9. Carr HY, Purcell EM. Effects of diffusion on free precession in nuclear magnetic resonance experiments. Phys Rev. 1954; 94:630–638.
Article10. Whittall KP, Mackay AL. Quantitative interpretation of NMR relaxation data. J Magn Reson. 1969; 84:134–152.
Article11. Coleman TF, Li Y. An interior trust region approach for nonlinear minimization subject to bounds. SIAM J Optim. 1996; 6:418–445.
Article12. Hurd RE, Yen YF, Mayer D, et al. Metabolic imaging in the anesthetized rat brain using hyperpolarized [1-13C] pyruvate and [1-13C] ethyl pyruvate. Magn Reson Med. 2010; 63:1137–1143.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Optimization of Scan Parameters for in vivo Hyperpolarized Carbon-13 Magnetic Resonance Spectroscopic Imaging
- Effect of Acaromyces Ingoldii Secondary Metabolites on the Growth of Brown-Rot (Gloeophyllum Trabeum) and White-Rot (Trametes Versicolor) Fungi
- Determination of Optimal Scan Time for the Measurement of Downstream Metabolites in Hyperpolarized 13C MRSI
- Alternating Acquisition Technique for Quantification of in vitro Hyperpolarized [1-13C] Pyruvate Metabolism
- Five New Wood Decay Fungi (Polyporales and Hymenochaetales) in Korea