Investig Magn Reson Imaging.
2016 Mar;20(1):53-60. 10.13104/imri.2016.20.1.53.
Alternating Acquisition Technique for Quantification of in vitro Hyperpolarized [1-13C] Pyruvate Metabolism
- Affiliations
-
- 1Department of Electrical and Electronic Engineering, Yonsei University, Seoul, Korea. donghyunkim@yonsei.ac.kr
- 2Center for Neuroscience Imaging Research, Institute for Basic Science, Sungkyunkwan University, Suwon, Korea.
- 3Department of Radiology, College of Medicine, Yonsei University, Seoul, Korea.
- KMID: 2161370
- DOI: http://doi.org/10.13104/imri.2016.20.1.53
Abstract
- PURPOSE
To develop a technique for quantifying the 13C-metabolites by performing frequency-selective hyperpolarized 13C magnetic resonance spectroscopy (MRS) in vitro which combines simple spectrally-selective excitation with spectrally interleaved acquisition.
METHODS
Numerical simulations were performed with varying noise level and K(p) values to compare the quantification accuracies of the proposed and the conventional methods. For in vitro experiments, a spectrally-selective excitation scheme was enabled by narrow-band radiofrequency (RF) excitation pulse implemented into a free-induction decay chemical shift imaging (FIDCSI) sequence. Experiments with LDH / NADH enzyme mixture were performed to validate the effectiveness of the proposed acquisition method. Also, a modified two-site exchange model was formulated for metabolism kinetics quantification with the proposed method.
RESULTS
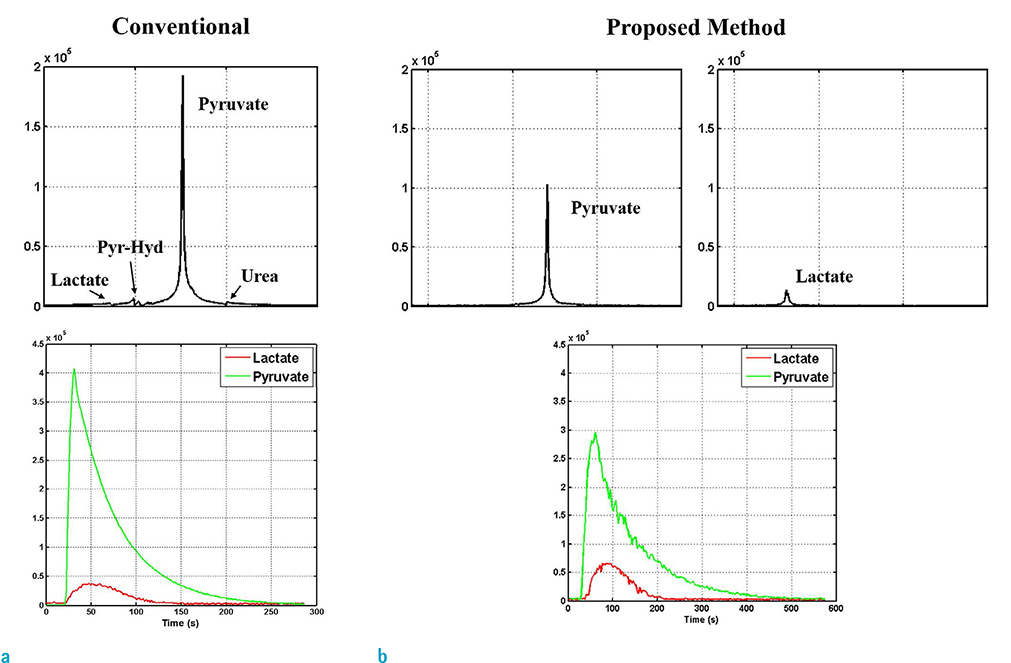
From the simulation results, significant increase of the lactate peak signal to noise ratio (PSNR) was observed. Also, the quantified K(p) value from the dynamic curves were more accurate in the case of the proposed acquisition method compared to the conventional non-selective excitation scheme. In vitro experiment results were in good agreement with the simulation results, also displaying increased PSNR for lactate. Fitting results using the modified two-site exchange model also showed expected results in agreement with the simulations.
CONCLUSION
A method for accurate quantification of hyperpolarized pyruvate and the downstream product focused on in vitro experiment was described. By using a narrow-band RF excitation pulse with alternating acquisition, different resonances were selectively excited with a different flip angle for increased PSNR while the hyperpolarized magnetization of the substrate can be minimally perturbed with a low flip angle. Baseline signals from neighboring resonances can be effectively suppressed to accurately quantify the metabolism kinetics.
Keyword
MeSH Terms
Figure
Reference
-
1. Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the 'phosphocreatine circuit' for cellular energy homeostasis. Biochem J. 1992; 281(Pt 1):21–40.2. Saunders DE, Howe FA, van den Boogaart A, Griffiths JR, Brown MM. Aging of the adult human brain: in vivo quantitation of metabolite content with proton magnetic resonance spectroscopy. J Magn Reson Imaging. 1999; 9:711–716.3. Wang Y, Li SJ. Differentiation of metabolic concentrations between gray matter and white matter of human brain by in vivo 1H magnetic resonance spectroscopy. Magn Reson Med. 1998; 39:28–33.4. Tan J, Bluml S, Hoang T, Dubowitz D, Mevenkamp G, Ross B. Lack of effect of oral choline supplement on the concentrations of choline metabolites in human brain. Magn Reson Med. 1998; 39:1005–1010.5. Baslow MH. N-acetylaspartate in the vertebrate brain: metabolism and function. Neurochem Res. 2003; 28:941–953.6. Birken DL, Oldendorf WH. N-acetyl-L-aspartic acid: a literature review of a compound prominent in 1H-NMR spectroscopic studies of brain. Neurosci Biobehav Rev. 1989; 13:23–31.7. Clark JB. N-acetyl aspartate: a marker for neuronal loss or mitochondrial dysfunction. Dev Neurosci. 1998; 20:271–276.8. Brambilla P, Perez J, Barale F, Schettini G, Soares JC. GABAergic dysfunction in mood disorders. Mol Psychiatry. 2003; 8:721–737.9. Oz G, Terpstra M, Tkac I, et al. Proton MRS of the unilateral substantia nigra in the human brain at 4 tesla: detection of high GABA concentrations. Magn Reson Med. 2006; 55:296–301.10. Ardenkjaer-Larsen JH, Fridlund B, Gram A, et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci U S A. 2003; 100:10158–10163.11. Nelson SJ, Kurhanewicz J, Vigneron DB, et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-(1)(3)C]pyruvate. Sci Transl Med. 2013; 5:198ra108.12. Park I, Larson PE, Tropp JL, et al. Dynamic hyperpolarized carbon-13 MR metabolic imaging of nonhuman primate brain. Magn Reson Med. 2014; 71:19–25.13. Lau AZ, Miller JJ, Robson MD, Tyler DJ. Cardiac perfusion imaging using hyperpolarized (13) c urea using flow sensitizing gradients. Magn Reson Med. 2016; 75:1474–1483.14. Albers MJ, Bok R, Chen AP, et al. Hyperpolarized 13C lactate, pyruvate, and alanine: noninvasive biomarkers for prostate cancer detection and grading. Cancer Res. 2008; 68:8607–8615.15. Harrison C, Yang C, Jindal A, et al. Comparison of kinetic models for analysis of pyruvate-to-lactate exchange by hyperpolarized 13 C NMR. NMR Biomed. 2012; 25:1286–1294.16. Atherton HJ, Schroeder MA, Dodd MS, et al. Validation of the in vivo assessment of pyruvate dehydrogenase activity using hyperpolarised 13C MRS. NMR Biomed. 2011; 24:201–208.17. Kettunen MI, Hu DE, Witney TH, et al. Magnetization transfer measurements of exchange between hyperpolarized [1-13C] pyruvate and [1-13C]lactate in a murine lymphoma. Magn Reson Med. 2010; 63:872–880.18. Xu T, Mayer D, Gu M, et al. Quantification of in vivo metabolic kinetics of hyperpolarized pyruvate in rat kidneys using dynamic 13C MRSI. NMR Biomed. 2011; 24:997–1005.19. Yen YF, Le Roux P, Mayer D, et al. T(2) relaxation times of (13)C metabolites in a rat hepatocellular carcinoma model measured in vivo using (13)C-MRS of hyperpolarized [1-(13)C]pyruvate. NMR Biomed. 2010; 23:414–423.20. Brindle KM. NMR methods for measuring enzyme kinetics in vivo. Prog NMR Spectrosc. 1988; 20:257–293.21. McConnell HM. Reaction rates by nuclear magnetic resonance. J Chem Phys. 1958; 28:430–431.22. Oppenheim AV, Willsky AS, Nawab SH. Signals and systems. 2nd ed. Upper Saddle River, NJ: Prentice Hall;1997. p. 957.23. Kazan SM, Reynolds S, Kennerley A, et al. Kinetic modeling of hyperpolarized (13)C pyruvate metabolism in tumors using a measured arterial input function. Magn Reson Med. 2013; 70:943–953.24. von Morze C, Larson PE, Hu S, et al. Imaging of blood flow using hyperpolarized [(13)C]urea in preclinical cancer models. J Magn Reson Imaging. 2011; 33:692–697.25. Zierhut ML, Yen YF, Chen AP, et al. Kinetic modeling of hyperpolarized 13C1-pyruvate metabolism in normal rats and TRAMP mice. J Magn Reson. 2010; 202:85–92.26. Santarelli MF, Positano V, Giovannetti G, et al. How the signal-to-noise ratio influences hyperpolarized 13C dynamic MRS data fitting and parameter estimation. NMR Biomed. 2012; 25:925–934.27. Menichetti L, Frijia F, Flori A, et al. Assessment of realtime myocardial uptake and enzymatic conversion of hyperpolarized [1-(1)(3)C]pyruvate in pigs using slice selective magnetic resonance spectroscopy. Contrast Media Mol Imaging. 2012; 7:85–94.28. Lee H, Lee J, Joe E, et al. Determination of optimal scan time for the measurement of downstream metabolites in hyperpolarized 13C MRSI. Investig Magn Reson Imaging. 2015; 19:212–217.29. Hill DK, Orton MR, Mariotti E, et al. Model free approach to kinetic analysis of real-time hyperpolarized 13C magnetic resonance spectroscopy data. PLoS One. 2013; 8:e71996.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Optimization of Scan Parameters for in vivo Hyperpolarized Carbon-13 Magnetic Resonance Spectroscopic Imaging
- Determination of Optimal Scan Time for the Measurement of Downstream Metabolites in Hyperpolarized 13C MRSI
- Dual Component Analysis for In Vivo T₂* Decay of Hyperpolarized ¹³C Metabolites
- Hyperpolarized [1- 13C]pyruvate Magnetic Resonance Spectroscopy Shows That Agmatine Increased Lactate Production in the Brain of Type 2 Diabetic Mice
- The in Vitro Maturation of the Mouse Oocyte