J Breast Cancer.
2015 Jun;18(2):149-159. 10.4048/jbc.2015.18.2.149.
Differences in Clinical Outcomes between Luminal A and B Type Breast Cancers according to the St. Gallen Consensus 2013
- Affiliations
-
- 1Department of Pathology, Inje University Busan Paik Hospital, Inje University College of Medicine, Busan, Korea. pathyoon@inje.ac.kr
- 2Department of General Surgery, Inje University Busan Paik Hospital, Inje University College of Medicine, Busan, Korea.
- 3Department of Clinical Pharmacology, Inje University Busan Paik Hospital, Inje University College of Medicine, Busan, Korea.
- KMID: 2374530
- DOI: http://doi.org/10.4048/jbc.2015.18.2.149
Abstract
- PURPOSE
Human epidermal growth factor receptor 2 (HER2)-positive luminal B type comprises estrogen receptor (ER)-positive and HER2-positive cancers, and HER2-negative luminal B type comprises ER-positive cancers showing a Ki-67 labeling index > or =14% or progesterone receptor (PR) expression of <20% according to the St. Gallen consensus 2013. The current study aimed to classify intrinsic subtypes according to the St. Gallen consensus 2013 and determine the differences in clinicopathological parameters and survival outcomes among the molecular types, especially among the luminal types.
METHODS
Assessment of molecular types was performed for 267 invasive ductal carcinomas. The differences in clinicopathological parameters, disease-free survival (DFS), and overall survival (OS) among the molecular types were analyzed.
RESULTS
The luminal B type was the most prevalent, at 44.9%, followed by the luminal A, triple-negative (including basal-like type), and HER2 type, at 21.7%, 18.7%, and 14.6%, respectively. There were statistically significant differences in size (p=0.003), nodal status (p=0.046), histologic grade (p<0.001), p53 (p<0.001) and cyclooxygenase 2 (COX-2) positivity (p=0.002), recurrence (p=0.001) and death rates (p=0.036), DFS (p=0.002), and OS (p=0.039) among the molecular types. Significant differences in size (p=0.009), nodal metastasis (p=0.019), histologic grade (p<0.001), p53 positivity (p=0.001), and PR expression (p<0.001) were noted between the luminal A and B types. Among the luminal B type cancers, the distributions of ER and PR scores showed significant differences (p=0.003, p=0.003). p53 positivity in the luminal B type cancers was related to shortened DFS (p=0.034). In luminal type cancers, COX-2 positivity was related to longer DFS (p=0.026).
CONCLUSION
Different management guidelines should be considered for the luminal A and luminal B breast cancer types. Positive p53 expression in luminal B type cancers and negative COX-2 expression in luminal type cancers seem to be related to poor clinical outcome.
MeSH Terms
-
Breast Neoplasms
Breast*
Carcinoma, Ductal
Consensus*
Cyclooxygenase 2
Disease-Free Survival
Estrogens
Humans
Ki-67 Antigen
Mortality
Neoplasm Metastasis
Phenobarbital*
Receptor, Epidermal Growth Factor
Receptors, Progesterone
Recurrence
Cyclooxygenase 2
Estrogens
Ki-67 Antigen
Phenobarbital
Receptor, Epidermal Growth Factor
Receptors, Progesterone
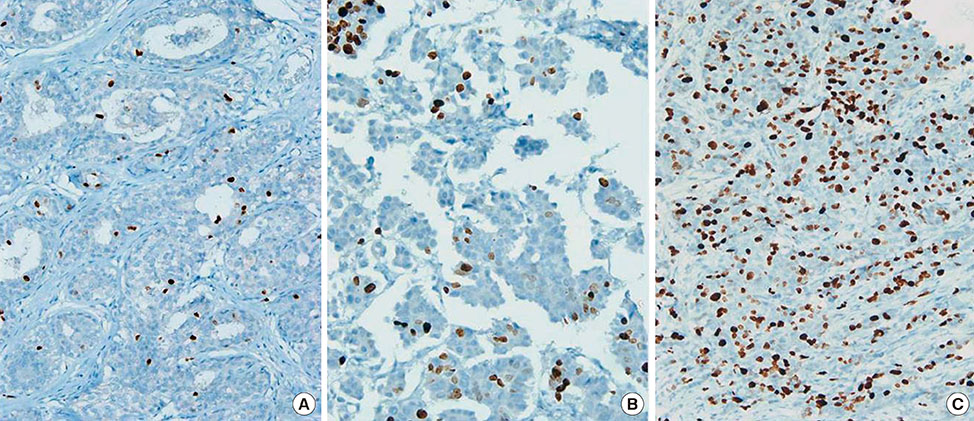
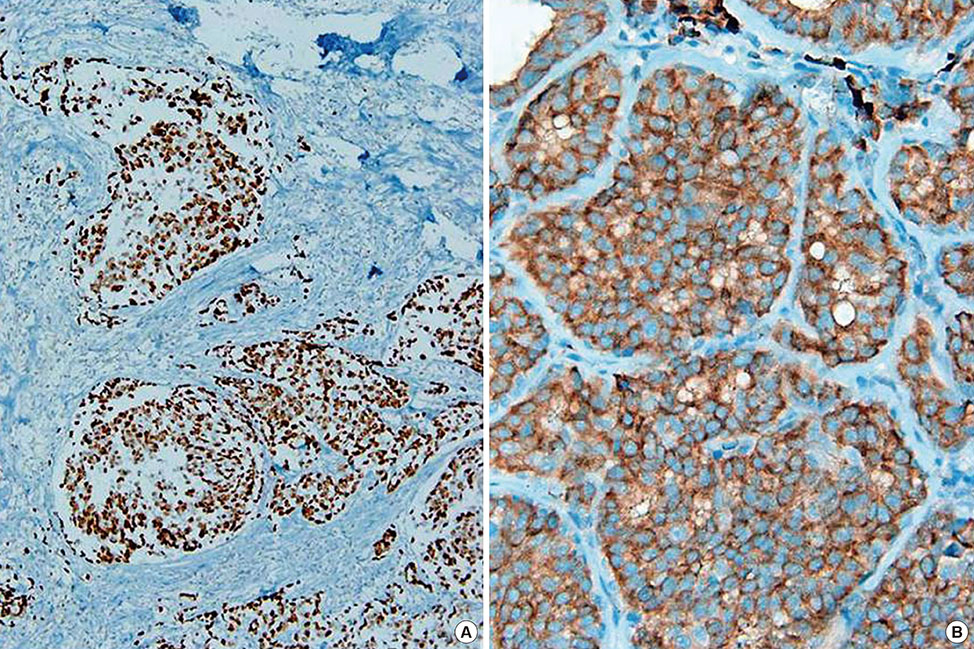
Figure
Reference
-
1. Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000; 406:747–752.
Article2. Wang GS, Zhu H, Bi SJ. Pathological features and prognosis of different molecular subtypes of breast cancer. Mol Med Rep. 2012; 6:779–782.
Article3. Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001; 98:10869–10874.
Article4. Cuzick J, Dowsett M, Pineda S, Wale C, Salter J, Quinn E, et al. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. J Clin Oncol. 2011; 29:4273–4278.
Article5. Braun L, Mietzsch F, Seibold P, Schneeweiss A, Schirmacher P, Chang-Claude J, et al. Intrinsic breast cancer subtypes defined by estrogen receptor signalling-prognostic relevance of progesterone receptor loss. Mod Pathol. 2013; 26:1161–1171.
Article6. Kim HS, Park I, Cho HJ, Gwak G, Yang K, Bae BN, et al. Analysis of the potent prognostic factors in luminal-type breast cancer. J Breast Cancer. 2012; 15:401–406.
Article7. Arpino G, Weiss H, Lee AV, Schiff R, De Placido S, Osborne CK, et al. Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst. 2005; 97:1254–1261.
Article8. Dowsett M, Nielsen TO, A'Hern R, Bartlett J, Coombes RC, Cuzick J, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011; 103:1656–1664.
Article9. Kang SH, Cheung KY, Kim YS. Correlation between hormonal receptor status and clinicopathologic factors with prognostic assesment in breast cancer. J Korean Surg Soc. 2003; 65:198–204.10. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, et al. Strategies for subtypes: dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011; 22:1736–1747.
Article11. Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013; 24:2206–2223.
Article12. Colozza M, Azambuja E, Cardoso F, Sotiriou C, Larsimont D, Piccart MJ. Proliferative markers as prognostic and predictive tools in early breast cancer: where are we now? Ann Oncol. 2005; 16:1723–1739.
Article13. Luporsi E, André F, Spyratos F, Martin PM, Jacquemier J, Penault-Llorca F, et al. Ki-67: level of evidence and methodological considerations for its role in the clinical management of breast cancer: analytical and critical review. Breast Cancer Res Treat. 2012; 132:895–915.
Article14. Ono M, Tsuda H, Yunokawa M, Yonemori K, Shimizu C, Tamura K, et al. Prognostic impact of Ki-67 labeling indices with 3 different cutoff values, histological grade, and nuclear grade in hormone-receptor-positive, HER2-negative, node-negative invasive breast cancers. Breast Cancer. 2015; 22:141–152.
Article15. Ahlin C, Aaltonen K, Amini RM, Nevanlinna H, Fjällskog ML, Blomqvist C. Ki67 and cyclin A as prognostic factors in early breast cancer. What are the optimal cut-off values? Histopathology. 2007; 51:491–498.
Article16. Polley MY, Leung SC, McShane LM, Gao D, Hugh JC, Mastropasqua MG, et al. An international Ki67 reproducibility study. J Natl Cancer Inst. 2013; 105:1897–1906.
Article17. Neri A, Marrelli D, Pedrazzani C, Caruso S, De Stefano A, Mariani F, et al. Prognostic relevance of proliferative activity evaluated by Mib-1 immunostaining in node negative breast cancer. Eur J Surg Oncol. 2008; 34:1299–1303.
Article18. Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999; 17:1474–1481.
Article19. Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013; 31:3997–4013.
Article20. Yemelyanova A, Vang R, Kshirsagar M, Lu D, Marks MA, Shih IeM, et al. Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Mod Pathol. 2011; 24:1248–1253.
Article21. Dhakal HP, Naume B, Synnestvedt M, Borgen E, Kaaresen R, Schlichting E, et al. Expression of cyclooxygenase-2 in invasive breast carcinomas and its prognostic impact. Histol Histopathol. 2012; 27:1315–1325.22. Maisonneuve P, Disalvatore D, Rotmensz N, Curigliano G, Colleoni M, Dellapasqua S, et al. Proposed new clinicopathological surrogate definitions of luminal A and luminal B (HER2-negative) intrinsic breast cancer subtypes. Breast Cancer Res. 2014; 16:R65.
Article23. Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009; 101:736–750.
Article24. Hida AI, Oshiro Y, Inoue H, Kawaguchi H, Yamashita N, Moriya T. Visual assessment of Ki67 at a glance is an easy method to exclude many luminal-type breast cancers from counting 1000 cells. Breast Cancer. 2015; 22:129–134.
Article25. Prat A, Cheang MC, Martín M, Parker JS, Carrasco E, Caballero R, et al. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. J Clin Oncol. 2013; 31:203–209.
Article26. Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol. 2003; 21:1973–1979.
Article27. Konecny G, Pauletti G, Pegram M, Untch M, Dandekar S, Aguilar Z, et al. Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J Natl Cancer Inst. 2003; 95:142–153.
Article28. Kim HJ, Cui X, Hilsenbeck SG, Lee AV. Progesterone receptor loss correlates with human epidermal growth factor receptor 2 overexpression in estrogen receptor-positive breast cancer. Clin Cancer Res. 2006; 12:1013s–1018s.
Article29. Coates AS, Millar EK, O'Toole SA, Molloy TJ, Viale G, Goldhirsch A, et al. Prognostic interaction between expression of p53 and estrogen receptor in patients with node-negative breast cancer: results from IBCSG Trials VIII and IX. Breast Cancer Res. 2012; 14:R143.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Highlights of 10th St. Gallen Breast Cancer Conference: Systemic Adjuvant Treatment
- Correlation of Molecular Subtypes of Invasive Ductal Carcinoma of Breast with Glucose Metabolism in FDG PET/CT: Based on the Recommendations of the St. Gallen Consensus Meeting 2013
- BCL2 as a Subtype-Specific Prognostic Marker for Breast Cancer
- Usefulness of Ki-67 as a prognostic factor in lymph node-negative breast cancer
- Prognostic Influence of BCL2 on Molecular Subtypes of Breast Cancer