J Breast Cancer.
2007 Mar;10(1):1-9. 10.4048/jbc.2007.10.1.1.
Highlights of 10th St. Gallen Breast Cancer Conference: Systemic Adjuvant Treatment
- Affiliations
-
- 1Department of Surgery, Yonsei University College of Medicine, Seoul Korea.
- 2Department of Surgery, Kyungpook National University, College of Medicine, Daegu, Korea. phy123@mail.knu.ac.kr
- KMID: 2174995
- DOI: http://doi.org/10.4048/jbc.2007.10.1.1
Abstract
- The 10th St. Gallen International Conference- Primary Therapy of Early Breast Cancer was held in March 2007. The St. Gallen Conferences has focused on reaching expert consensus for patient treatment selection. Three categories were affirmed by responsiveness of endocrine treatment- endocrine responsive, endocrine responsive uncertain, endocrine non-responsive. Risk assessment will be similar than previous meeting (9th meeting) - low, intermediate, and high risk categories. The Panel recommended that patients be offered endocrine therapy or trastuzumab according to endocrine responsiveness or HER2 status. Chemotherapy offered to patients according to risk assessment. For patients with endocrine responsive and HER2 negative, selection of patient for chemotherapy is major challenge. The Panel of Expert attempted to answer many questions- endocrine therapy, chemotherapy, anti-HER2 therapy, and radiation therapy. This report focused on new information related to the best use of endocrine therapy and chemotherapy.
MeSH Terms
Figure
Reference
-
1. Goldhirsch A, Glick JH, Gelber RD, Coates AS, Thurlimann B, Senn HJ, et al. Meeting highlight: International expert consensus on the primary therapy oh early breast cancer 2005. Ann Oncol. 2005. 16:1569–1583.
Article2. ATAC Trialists' Group. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005. 365:60–62.3. Coates AS, Keshaviah A, Thürlimann B, Mouridsen H, Mauriac L, Forbes JF, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J Clin Oncol. 2007. 25:486–492.
Article4. Jakesz R, Jonat W, Gnant M, Mittlboeck M, Greil R, Tausch C, et al. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years' adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet. 2005. 366:455–462.
Article5. Boccardo F, Rubagotti A, Puntoni M, Guglielmini P, Amoroso D, Fini A, et al. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer: preliminary results of the Italian Tamoxifen Anastrozole Trial. J Clin Oncol. 2005. 23:5138–5147.
Article6. Coombes RC, Kilburn LS, Snowdon CF, Paridaens R, Coleman RE, Jones SE, et al. Survival and safety of exemestane versus tamoxifen after 2-3 years' tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet. 2007. 369:559–570.
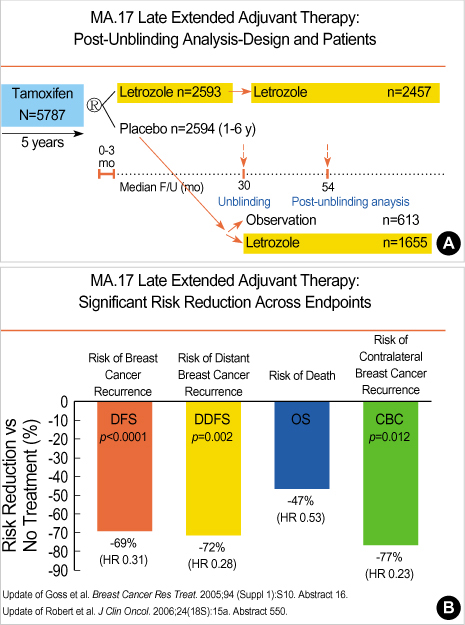
Article7. Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst. 2005. 97:1262–1271.
Article8. Cuzick J, Sasieni P, Howell A. Should aromatase inhibitors be used as initial adjuvant treatment or sequenced after tamoxifen? Br J Cancer. 2006. 94:460–464.
Article9. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005. 365:1687–1717.10. Kaufmann M, Jonat W, Blamey R, Cuzick J, Namer M, Fogelman I, et al. Survival analyses from the ZEBRA study. goserelin (Zoladex) versus CMF in premenopausal women with node-positive breast cancer. Eur J Cancer. 2003. 39:1711–1717.11. International Breast Cancer Study Group (IBCSG). Adjuvant chemotherapy followed by goserelin versus either modality alone for premenopausal lymph node-negative breast cancer: a randomized trial. J Natl Cancer Inst. 2003. 95:1833–1846.12. Jakesz R, Hausmaninger H, Kubista E, Gnant M, Menzel C, Bauernhofer T, et al. Randomized adjuvant trial of tamoxifen and goserelin versus cyclophosphamide, methotrexate, and fluorouracil: evidence for the superiority of treatment with endocrine blockade in premenopausal patients with hormone-responsive breast cancer--Austrian Breast and Colorectal Cancer Study Group Trial 5. J Clin Oncol. 2002. 20:4621–4627.
Article13. Davidson NE, O'Neill AM, Vukov AM, Osborne CK, Martino S, White DR, et al. Chemoendocrine therapy for premenopausal women with axillary lymph node- positive, steroid hormone receptor-positive breast cancer: Results From INT 0101 (E5188). J Clin Oncol. 2005. 23:5973–5982.
Article14. Baum M, Hackshaw A, Houghton J, Rutqvist , Fornander T, Nordenskjold B, et al. Adjuvant goserelin in pre-menopausal patients with early breast cancer: Results from the ZIPP study. Eur J Cancer. 2006. 42:895–904.
Article15. Buyse M, Loi S, van't Veer L, Viale G, Delorenzi M, Glas AM, et al. Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J Natl Cancen Inst. 2006. 98:1183–1192.
Article16. Paik S, Tang G, Shak S. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006. 24:2019–2027.
Article17. Kaklamani V. A genetic signature can predict prognosis and response to therapy in breast cancer: Oncotype DX. Expert Rev Mol Diagn. 2006. 6:803–809.
Article18. Trudeau M, Charbonneau F, Gelmon K, Laing K, Latreille J, Mackey J, et al. Selection of adjuvant chemotherapy for treatment of node-positive breast cancer. Lancet Oncol. 2005. 6:886–898.
Article19. Verma S, Trudeau M, Dranitsaris G, Clemons M, Joy AA, MacKey JR. What is the best chemotherapy treatment option for anthracycline and taxane pretreated metastatic breast cancer? J Clin Oncol. 2005. 23:6260–6261.
Article20. Martin M, Pienkowski T, Mackey J, Pawlicki M, Guastalla JP, Weaver C, et al. Adjuvant docetaxel for node-positive breast cancer. New Engl J Med. 2005. 352:2302–2313.
Article21. Campone M, Fumoleau P, Bourbouloux E, Kerbrat P, Roche H. Taxanes in adjuvant breast cancer setting: which standard in Europe? Crit Rev Oncol Hematol. 2005. 55:167–175.
Article22. Roche H, Fumoleau P, Spielmann M, Canon JL, Delozier T, Serin D, et al. Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: the FNCLCC PACS 01 Trial. J Clin Oncol. 2006. 24:5664–5671.
Article23. Citron ML, Berry DA, Cirrincione C, Hudis C, Winer EP, Gradishar WJ, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003. 21:1431–1439.
Article24. Hudis C. The best use of adjuvant chemotherapy: new drugs and new use of "old" drugs. Breast. 2005. 14:570–575.
Article25. Hudis CA, Winer EP. Cancer and leukemia group B breast committee: decades of progress and plans for the future. Clin Cancer Res. 2006. 12:3576–3580.
Article26. Moore HC, Green SJ, Gralow JR, Bearman SI, Lew D, Barlow WE, et al. Intensive dose-dense compared with high-dose adjuvant chemotherapy for high-risk operable breast cancer: Southwest Oncology Group/Intergroup study 9623. J Clin Oncol. 2007. 25:1677–1682.
Article27. Holmes FA, Jones SE, O'Shaughnessy J, Vukelja S, George T, Savin M, et al. Comparable efficacy and safety profiles of once-per-cycle pegfilgrastim and daily injection filgrastim in chemotherapy-induced neutropenia: a multicenter dose-finding study in women with breast cancer. Ann Oncol. 2002. 13:903–909.
Article28. Jones SE, Clark G, Koleszar S, Ethington G, Mennel R, Paulson S, et al. Adjuvant chemotherapy with doxorubicin and cyclophosphamide in women with rapidly proliferating node-negative breast cancer. Clin Breast Cancer. 2002. 3:147–152.
Article29. Jones SE, Savin MA, Holmes FA, O'Shaughnessy JA, Blum JL, Vukelja S, et al. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol. 2006. 24:5381–5387.
Article30. Gluck S. Adjuvant chemotherapy for early breast cancer: optimal use of epirubicin. Oncologist. 2005. 10:780–791.
Article