Yonsei Med J.
2017 Jan;58(1):19-26. 10.3349/ymj.2017.58.1.19.
Detection of Circulating Tumor Cells in Breast Cancer Patients Using Cytokeratin-19 Real-Time RT-PCR
- Affiliations
-
- 1Department of Surgery, Yonsei University College of Medicine, Seoul, Korea. skim@yuhs.ac
- 2Avison Biomedical Research Center, Yonsei University College of Medicine, Seoul, Korea.
- 3Division of Medical Oncology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. oncosohn@yuhs.ac
- KMID: 2374184
- DOI: http://doi.org/10.3349/ymj.2017.58.1.19
Abstract
- PURPOSE
The roles of circulating tumor cells (CTCs) as predictive and prognostic factors, as well as key mediators in the metastatic cascade, have been investigated. This study aimed to validate a method to quantify CTCs in peripheral blood using a real-time reverse transcriptase polymerase chain reaction (RT-PCR) assay for cytokeratin (CK)-19 and to evaluate the utility of this assay in detecting CTCs in breast cancer patients.
MATERIALS AND METHODS
Real-time monitoring PCR of fluorescently labeled specific hybridization probes for CK-19 mRNA was established. Peripheral blood samples from 30 healthy donors, 69 patients with early breast cancer, 47 patients with locally advanced breast cancer, and 126 patients with metastatic breast cancer were prospectively obtained and analyzed for CTC detection.
RESULTS
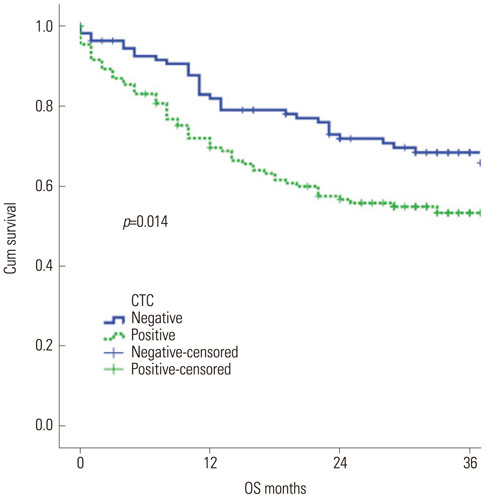
CK-19 mRNA was not detectable in healthy subjects using the real-time RT-PCR method. The detection rates of CK-19 mRNA in breast cancer patients were 47.8% for early breast cancer (33/69), 46.8% for locally advanced breast cancer (22/47), and 61.1% for metastatic breast cancer (77/129). The detection rate of CK-19-positive CTCs in metastatic disease was slightly higher than early or locally advanced breast cancer; however, the detection rate according to disease burden was not statistically different (p=0.097). The detection rate was higher in patients with pleural metastasis (p=0.045). CTC detection was associated with poor survival (p=0.014).
CONCLUSION
A highly specific and sensitive CK-19 mRNA-based method to detect CTCs in peripheral blood in breast cancer patients can be used in further prospective studies to evaluate the predictive and prognostic importance of CTCs.
Keyword
MeSH Terms
-
Biomarkers, Tumor/*blood
Breast Neoplasms/blood/*pathology
Female
Humans
Keratin-19/*blood/genetics
*Neoplastic Cells, Circulating
Prognosis
Prospective Studies
RNA, Messenger/*blood
Real-Time Polymerase Chain Reaction
*Reverse Transcriptase Polymerase Chain Reaction/methods
Biomarkers, Tumor
Keratin-19
RNA, Messenger
Figure
Reference
-
1. Fehm T, Müller V, Alix-Panabières C, Pantel K. Micrometastatic spread in breast cancer: detection, molecular characterization and clinical relevance. Breast Cancer Res. 2008; 10:Suppl 1. S1.
Article2. Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004; 351:781–791.
Article3. Xenidis N, Ignatiadis M, Apostolaki S, Perraki M, Kalbakis K, Agelaki S, et al. Cytokeratin-19 mRNA-positive circulating tumor cells after adjuvant chemotherapy in patients with early breast cancer. J Clin Oncol. 2009; 27:2177–2184.
Article4. Cristofanilli M, Hayes DF, Budd GT, Ellis MJ, Stopeck A, Reuben JM, et al. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol. 2005; 23:1420–1430.
Article5. Dawood S, Broglio K, Valero V, Reuben J, Handy B, Islam R, et al. Circulating tumor cells in metastatic breast cancer: from prognostic stratification to modification of the staging system? Cancer. 2008; 113:2422–2430.
Article6. Pantel K, Alix-Panabières C, Riethdorf S. Cancer micrometastases. Nat Rev Clin Oncol. 2009; 6:339–351.
Article7. Paterlini-Brechot P, Benali NL. Circulating tumor cells (CTC) detection: clinical impact and future directions. Cancer Lett. 2007; 253:180–204.
Article8. Stathopoulou A, Gizi A, Perraki M, Apostolaki S, Malamos N, Mavroudis D, et al. Real-time quantification of CK-19 mRNA-positive cells in peripheral blood of breast cancer patients using the lightcycler system. Clin Cancer Res. 2003; 9:5145–5151.9. Ring AE, Zabaglo L, Ormerod MG, Smith IE, Dowsett M. Detection of circulating epithelial cells in the blood of patients with breast cancer: comparison of three techniques. Br J Cancer. 2005; 92:906–912.
Article10. Van der Auwera I, Peeters D, Benoy IH, Elst HJ, Van Laere SJ, Prové A, et al. Circulating tumour cell detection: a direct comparison between the CellSearch System, the AdnaTest and CK-19/mammaglobin RT-PCR in patients with metastatic breast cancer. Br J Cancer. 2010; 102:276–284.
Article11. Zhao S, Liu Y, Zhang Q, Li H, Zhang M, Ma W, et al. The prognostic role of circulating tumor cells (CTCs) detected by RT-PCR in breast cancer: a meta-analysis of published literature. Breast Cancer Res Treat. 2011; 130:809–816.
Article12. Stathopoulou A, Ntoulia M, Perraki M, Apostolaki S, Mavroudis D, Malamos N, et al. A highly specific real-time RT-PCR method for the quantitative determination of CK-19 mRNA positive cells in peripheral blood of patients with operable breast cancer. Int J Cancer. 2006; 119:1654–1659.
Article13. Kahn HJ, Yang LY, Blondal J, Lickley L, Holloway C, Hanna W, et al. RT-PCR amplification of CK19 mRNA in the blood of breast cancer patients: correlation with established prognostic parameters. Breast Cancer Res Treat. 2000; 60:143–151.
Article14. Stathopoulou A, Vlachonikolis I, Mavroudis D, Perraki M, Kouroussis Ch, Apostolaki S, et al. Molecular detection of cytokeratin-19-positive cells in the peripheral blood of patients with operable breast cancer: evaluation of their prognostic significance. J Clin Oncol. 2002; 20:3404–3412.
Article15. Xenidis N, Vlachonikolis I, Mavroudis D, Perraki M, Stathopoulou A, Malamos N, et al. Peripheral blood circulating cytokeratin-19 mRNA-positive cells after the completion of adjuvant chemotherapy in patients with operable breast cancer. Ann Oncol. 2003; 14:849–855.
Article16. Xenidis N, Perraki M, Kafousi M, Apostolaki S, Bolonaki I, Stathopoulou A, et al. Predictive and prognostic value of peripheral blood cytokeratin-19 mRNA-positive cells detected by real-time polymerase chain reaction in node-negative breast cancer patients. J Clin Oncol. 2006; 24:3756–3762.
Article17. Xenidis N, Markos V, Apostolaki S, Perraki M, Pallis A, Sfakiotaki G, et al. Clinical relevance of circulating CK-19 mRNA-positive cells detected during the adjuvant tamoxifen treatment in patients with early breast cancer. Ann Oncol. 2007; 18:1623–1631.
Article18. Ignatiadis M, Perraki M, Apostolaki S, Politaki E, Xenidis N, Kafousi M, et al. Molecular detection and prognostic value of circulating cytokeratin-19 messenger RNA-positive and HER2 messenger RNA-positive cells in the peripheral blood of women with early-stage breast cancer. Clin Breast Cancer. 2007; 7:883–889.
Article19. Ignatiadis M, Xenidis N, Perraki M, Apostolaki S, Politaki E, Kafousi M, et al. Different prognostic value of cytokeratin-19 mRNA positive circulating tumor cells according to estrogen receptor and HER2 status in early-stage breast cancer. J Clin Oncol. 2007; 25:5194–5202.
Article20. Ignatiadis M, Kallergi G, Ntoulia M, Perraki M, Apostolaki S, Kafousi M, et al. Prognostic value of the molecular detection of circulating tumor cells using a multimarker reverse transcription-PCR assay for cytokeratin 19, mammaglobin A, and HER2 in early breast cancer. Clin Cancer Res. 2008; 14:2593–2600.
Article21. Chen Y, Zou TN, Wu ZP, Zhou YC, Gu YL, Liu X, et al. Detection of cytokeratin 19, human mammaglobin, and carcinoembryonic antigen-positive circulating tumor cells by three-marker reverse transcription-PCR assay and its relation to clinical outcome in early breast cancer. Int J Biol Markers. 2010; 25:59–68.
Article22. Benoy IH, Elst H, Philips M, Wuyts H, Van Dam P, Scharpé S, et al. Real-time RT-PCR detection of disseminated tumour cells in bone marrow has superior prognostic significance in comparison with circulating tumour cells in patients with breast cancer. Br J Cancer. 2006; 94:672–680.
Article23. Daskalaki A, Agelaki S, Perraki M, Apostolaki S, Xenidis N, Stathopoulos E, et al. Detection of cytokeratin-19 mRNA-positive cells in the peripheral blood and bone marrow of patients with operable breast cancer. Br J Cancer. 2009; 101:589–597.
Article24. Grünewald K, Haun M, Urbanek M, Fiegl M, Müller-Holzner E, Gunsilius E, et al. Mammaglobin gene expression: a superior marker of breast cancer cells in peripheral blood in comparison to epidermal-growth-factor receptor and cytokeratin-19. Lab Invest. 2000; 80:1071–1077.
Article25. Cha YJ, Jung WH, Cho NH, Koo JS. Expression of sarcosine metabolism-related proteins in invasive lobular carcinoma: comparison to invasive ductal carcinoma. Yonsei Med J. 2015; 56:598–607.
Article26. Criscitiello C, Sotiriou C, Ignatiadis M. Circulating tumor cells and emerging blood biomarkers in breast cancer. Curr Opin Oncol. 2010; 22:552–558.
Article27. Thurm H, Ebel S, Kentenich C, Hemsen A, Riethdorf S, Coith C, et al. Rare expression of epithelial cell adhesion molecule on residual micrometastatic breast cancer cells after adjuvant chemotherapy. Clin Cancer Res. 2003; 9:2598–2604.28. Rack B, Schindlbeck C, Jückstock J, Andergassen U, Hepp P, Zwingers T, et al. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst. 2014; 05. 15. [Epub]. DOI: 10.1093/jnci/dju066.
Article29. Soltani S, Mokarian F, Panjehpour M. The expression of CK-19 gene in circulating tumor cells of blood samples of metastatic breast cancer women. Res Pharm Sci. 2015; 10:485–496.30. Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006; 12(14 Pt 1):4218–4224.
Article31. de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008; 14:6302–6309.
Article32. Tewes M, Aktas B, Welt A, Mueller S, Hauch S, Kimmig R, et al. Molecular profiling and predictive value of circulating tumor cells in patients with metastatic breast cancer: an option for monitoring response to breast cancer related therapies. Breast Cancer Res Treat. 2009; 115:581–590.
Article33. Hyun KA, Koo GB, Han H, Sohn J, Choi W, Kim SI, et al. Epithelial-to-mesenchymal transition leads to loss of EpCAM and different physical properties in circulating tumor cells from metastatic breast cancer. Oncotarget. 2016; 7:24677–24687.
Article34. Lohr JG, Adalsteinsson VA, Cibulskis K, Choudhury AD, Rosenberg M, Cruz-Gordillo P, et al. Whole-exome sequencing of circulating tumor cells provides a window into metastatic prostate cancer. Nat Biotechnol. 2014; 32:479–484.
Article35. Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014; 158:1110–1122.
Article36. Yu M, Bardia A, Aceto N, Bersani F, Madden MW, Donaldson MC, et al. Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science. 2014; 345:216–220.
Article37. Hyun KA, Kwon K, Han H, Kim SI, Jung HI. Microfluidic flow fractionation device for label-free isolation of circulating tumor cells (CTCs) from breast cancer patients. Biosens Bioelectron. 2013; 40:206–212.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum to “Detection of Circulating Tumor Cells in Breast Cancer Patients Using Cytokeratin-19 Real-Time RT-PCR†by Park HS, et al. (Yonsei Med J 2017;58:19-26.)
- The detection of circulating breast cancer cells in peripheral blood by reverse transcriptase-polymerase chain reaction
- Detection of Axillary Lymph Node Micrometastases in Breast Cancer Using RT-PCR Comparison the Results of MUC1, Cytokeratin 19
- Detection of Axillary Lymph Node Micrometastases in Breast Cancer Using RT-PCR: Comparison the Results of MUC1, Cytokeratin 19
- Detection of Micrometastasis in Peripheral Blood of Breast Cancer Patients Using RT- PCR Assay: Comparison of MUC1, CK19 and hMMG