Yonsei Med J.
2016 Sep;57(5):1095-1105. 10.3349/ymj.2016.57.5.1095.
Development of Advanced Atherosclerotic Plaque by Injection of Inflammatory Proteins in a Rabbit Iliac Artery Model
- Affiliations
-
- 1Cardiology Division, Severance Cardiovascular Hospital, Yonsei University College of Medicine, Seoul, Korea. shpark0530@yuhs.ac
- 2Cardiovascular Product Evaluation Center, Yonsei University College of Medicine, Seoul, Korea.
- 3Cardiovascular Research Institute, Yonsei University College of Medicine, Seoul, Korea.
- 4Graduate Program in Science for Aging, Yonsei University, Seoul, Korea.
- 5Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
- 6Severance Biomedical Science Institute, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2374153
- DOI: http://doi.org/10.3349/ymj.2016.57.5.1095
Abstract
- PURPOSE
Appropriate animal models of atherosclerotic plaque are crucial to investigating the pathophysiology of atherosclerosis, as well as for the evaluation of the efficacy and safety of vascular devices. We aimed to develop a novel animal model that would be suitable for the study of advanced atherosclerotic lesions in vivo.
MATERIALS AND METHODS
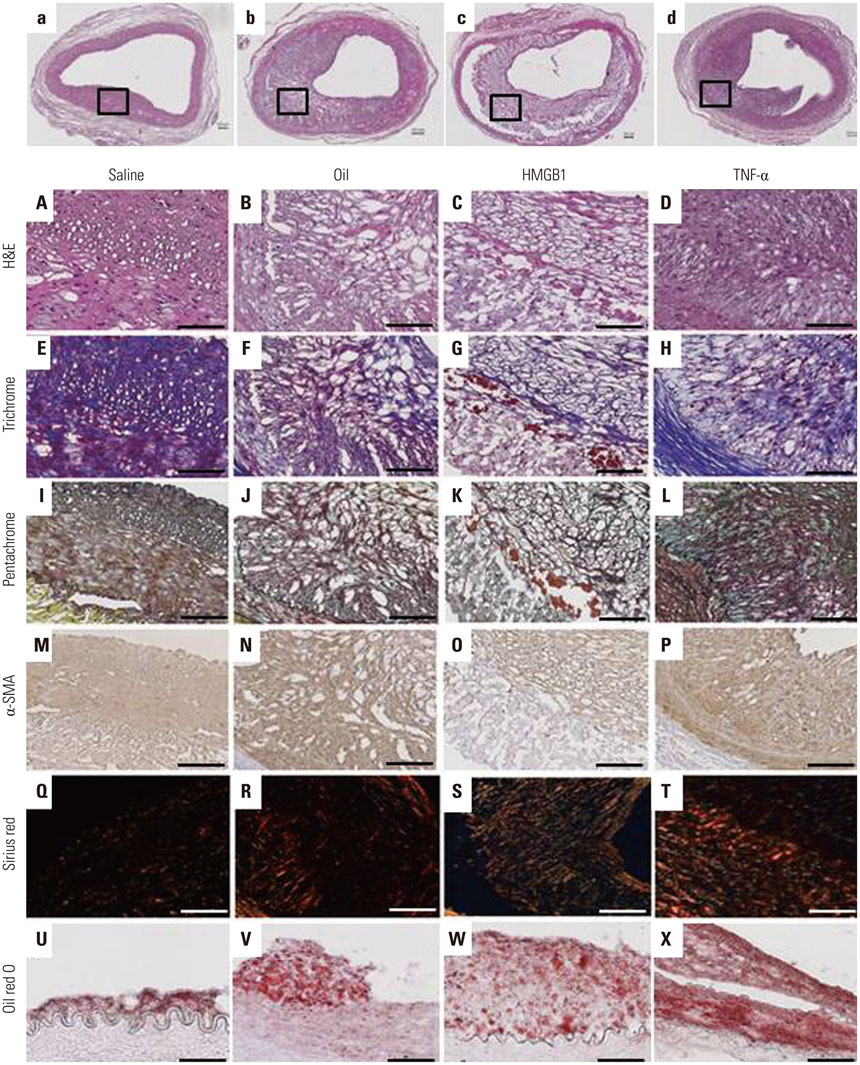
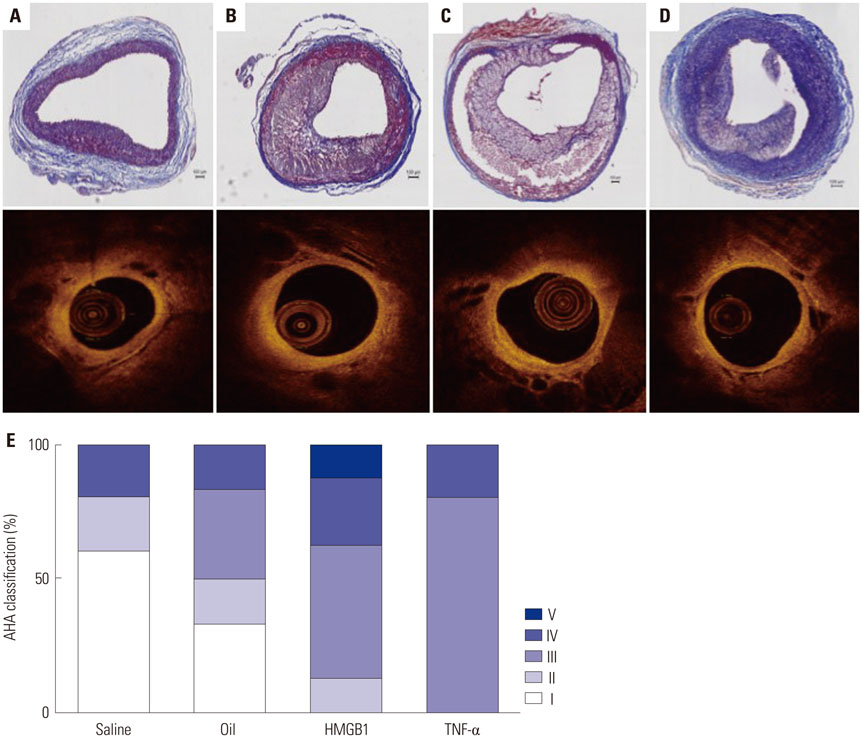
Atherosclerotic plaque was induced in 24 iliac arteries from 12 rabbits by combining a high cholesterol diet, endothelial denudation, and injection into the vessel wall with either saline (n=5), olive oil (n=6), or inflammatory proteins [n=13, high-mobility group protein B1 (HMGB1) n=8 and tumor necrosis factor (TNF)-α n=5] using a Cricket™ Micro-infusion catheter. Optical coherence tomography (OCT) was performed to detect plaque characteristics after 4 weeks, and all tissues were harvested for histological evaluation.
RESULTS
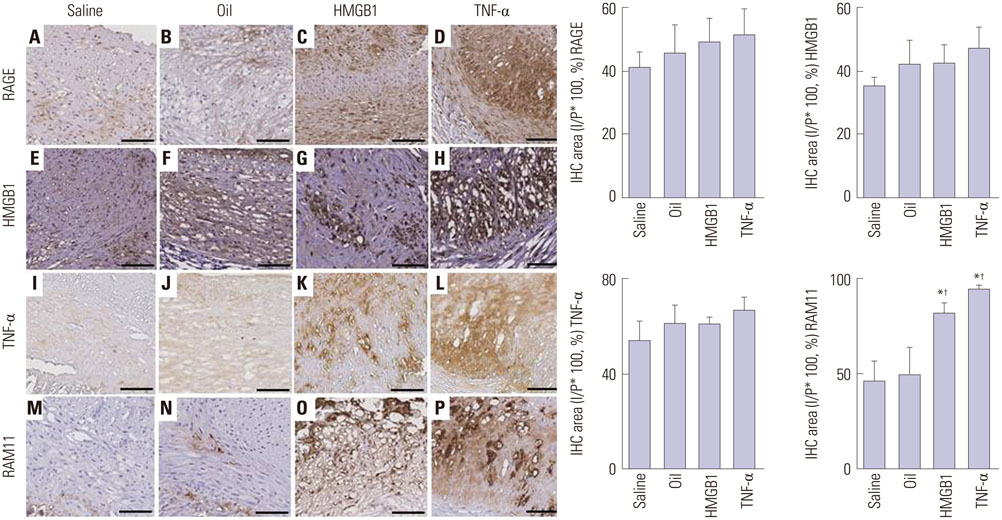
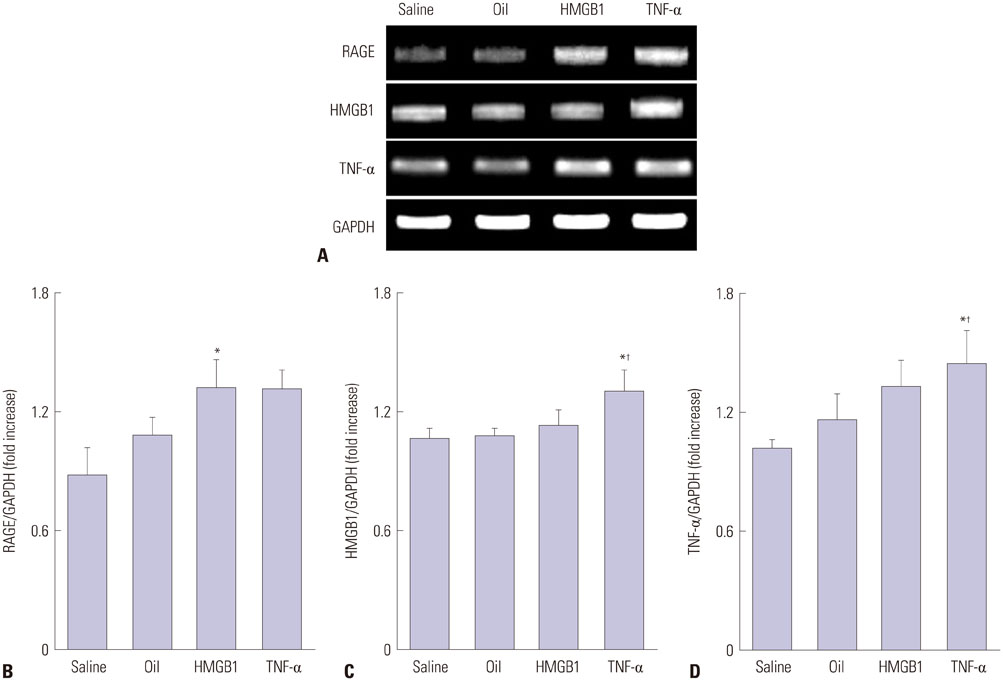
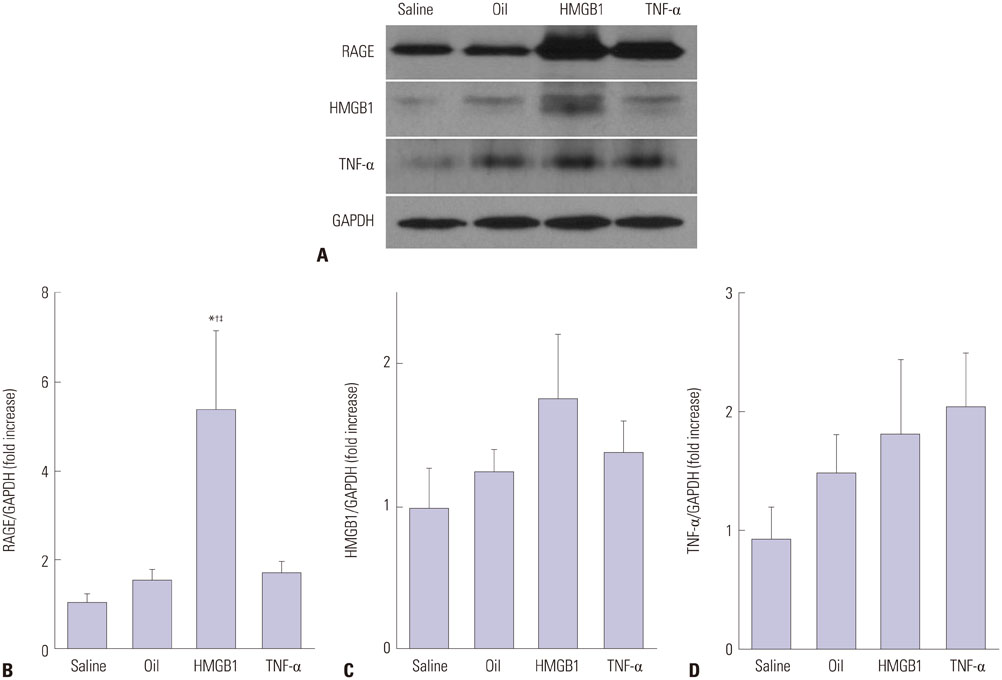
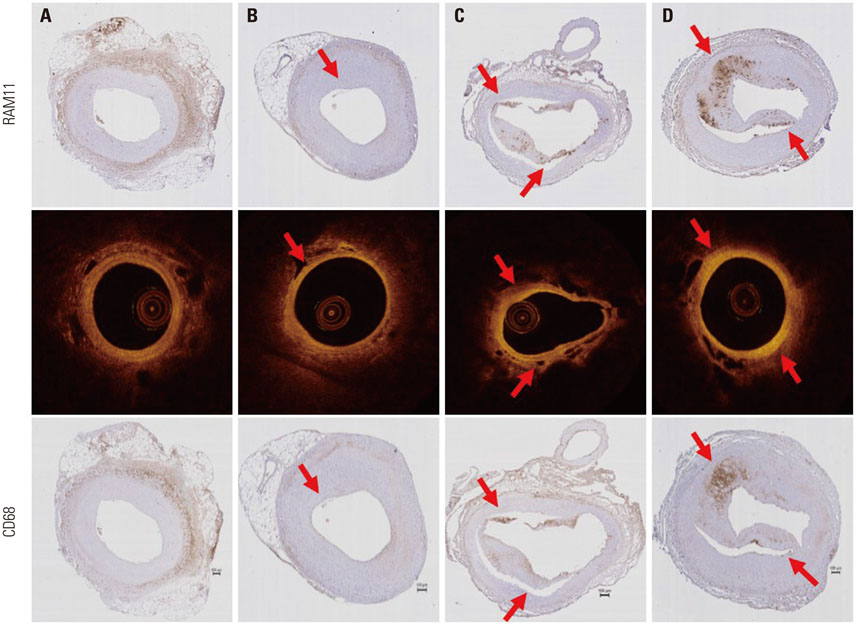
Advanced plaque was more frequently observed in the group injected with inflammatory proteins. Macrophage infiltration was present to a higher degree in the HMGB1 and TNF-α groups, compared to the oil or saline group (82.1±5.1% and 94.6±2.2% compared to 49.6±14.0% and 46.5±9.6%, p-value<0.001), using RAM11 antibody staining. On OCT, lipid rich plaques were more frequently detected in the inflammatory protein group [saline group: 2/5 (40%), oil group: 3/5 (50%), HMGB1 group: 6/8 (75%), and TNF-α group: 5/5 (100%)].
CONCLUSION
These data indicate that this rabbit model of atherosclerotic lesion formation via direct injection of pro-inflammatory proteins into the vessel wall is useful for in vivo studies investigating atherosclerosis.
MeSH Terms
-
Animals
Cholesterol, Dietary/administration & dosage
*Disease Models, Animal
Endothelium/surgery
HMGB1 Protein/*adverse effects
Iliac Artery/diagnostic imaging/pathology/surgery
Injections, Intra-Arterial
Macrophages
Male
Olive Oil/adverse effects
Plaque, Atherosclerotic/*chemically induced/diagnostic imaging/pathology
Rabbits
Sodium Chloride/adverse effects
Tomography, Optical Coherence
Tumor Necrosis Factor-alpha/*adverse effects
Cholesterol, Dietary
HMGB1 Protein
Olive Oil
Sodium Chloride
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Arbab-Zadeh A, Nakano M, Virmani R, Fuster V. Acute coronary events. Circulation. 2012; 125:1147–1156.
Article2. Otsuka F, Joner M, Prati F, Virmani R, Narula J. Clinical classification of plaque morphology in coronary disease. Nat Rev Cardiol. 2014; 11:379–389.
Article3. Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006; 47:8 Suppl. C13–C18.
Article4. Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014; 114:1852–1866.
Article5. Granada JF, Wallace-Bradley D, Win HK, Alviar CL, Builes A, Lev EI, et al. In vivo plaque characterization using intravascular ultrasound-virtual histology in a porcine model of complex coronary lesions. Arterioscler Thromb Vasc Biol. 2007; 27:387–393.
Article6. Phinikaridou A, Hallock KJ, Qiao Y, Hamilton JA. A robust rabbit model of human atherosclerosis and atherothrombosis. J Lipid Res. 2009; 50:787–797.
Article7. Granada JF, Kaluza GL, Wilensky RL, Biedermann BC, Schwartz RS, Falk E. Porcine models of coronary atherosclerosis and vulnerable plaque for imaging and interventional research. EuroIntervention. 2009; 5:140–148.
Article8. Fan J, Kitajima S, Watanabe T, Xu J, Zhang J, Liu E, et al. Rabbit models for the study of human atherosclerosis: from pathophysiological mechanisms to translational medicine. Pharmacol Ther. 2015; 146:104–119.
Article9. Granada JF, Moreno PR, Burke AP, Schulz DG, Raizner AE, Kaluza GL. Endovascular needle injection of cholesteryl linoleate into the arterial wall produces complex vascular lesions identifiable by intravascular ultrasound: early development in a porcine model of vulnerable plaque. Coron Artery Dis. 2005; 16:217–224.
Article10. Tellez A, Schuster DS, Alviar C, López-Berenstein G, Sanguino A, Ballantyne C, et al. Intramural coronary lipid injection induces atheromatous lesions expressing proinflammatory chemokines: implications for the development of a porcine model of atherosclerosis. Cardiovasc Revasc Med. 2011; 12:304–311.
Article11. Ridker PM, Lüscher TF. Anti-inflammatory therapies for cardiovascular disease. Eur Heart J. 2014; 35:1782–1791.
Article12. Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W Jr, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol. 1995; 15:1512–1531.
Article13. Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012; 59:1058–1072.14. Tearney GJ. OCT imaging of macrophages: a bright spot in the study of inflammation in human atherosclerosis. JACC Cardiovasc Imaging. 2015; 8:73–75.15. McKellar GE, McCarey DW, Sattar N, McInnes IB. Role for TNF in atherosclerosis? Lessons from autoimmune disease. Nat Rev Cardiol. 2009; 6:410–417.
Article16. Park S, Yoon SJ, Tae HJ, Shim CY. RAGE and cardiovascular disease. Front Biosci (Landmark Ed). 2011; 16:486–497.
Article17. Hirata Y, Kurobe H, Higashida M, Fukuda D, Shimabukuro M, Tanaka K, et al. HMGB1 plays a critical role in vascular inflammation and lesion formation via toll-like receptor 9. Atherosclerosis. 2013; 231:227–233.
Article18. Porto A, Palumbo R, Pieroni M, Aprigliano G, Chiesa R, Sanvito F, et al. Smooth muscle cells in human atherosclerotic plaques secrete and proliferate in response to high mobility group box 1 protein. FASEB J. 2006; 20:2565–2566.
Article19. Kim JS, Afari ME, Ha J, Tellez A, Milewski K, Conditt G, et al. Neointimal patterns obtained by optical coherence tomography correlate with specific histological components and neointimal proliferation in a swine model of restenosis. Eur Heart J Cardiovasc Imaging. 2014; 15:292–298.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Association with inflammatory cells and apolipoproteins to the progression of atherosclerosis
- Development of a Rabbit Model for a Preclinical Comparison of Coronary Stent Types In-Vivo
- Nuclear Molecular Imaging for Vulnerable Atherosclerotic Plaques
- Diagnostic and Therapeutic Approach of Carotid and Cerebrovascular Plaque on the Basis of Vessel Imaging
- Endovascular Management for Chronic Steno-occlusion of Iliac and Femoral Arteries