Korean Circ J.
2013 Nov;43(11):713-722. 10.4070/kcj.2013.43.11.713.
Development of a Rabbit Model for a Preclinical Comparison of Coronary Stent Types In-Vivo
- Affiliations
-
- 1Innovative Research Institute for Cell Therapy, Seoul National University Hospital, Seoul, Korea. usahyosoo@gmail.com
- 2World Class University Program, Department of Molecular Medicine and Biopharmaceutical Sciences, Seoul National University, Seoul, Korea.
- KMID: 2224801
- DOI: http://doi.org/10.4070/kcj.2013.43.11.713
Abstract
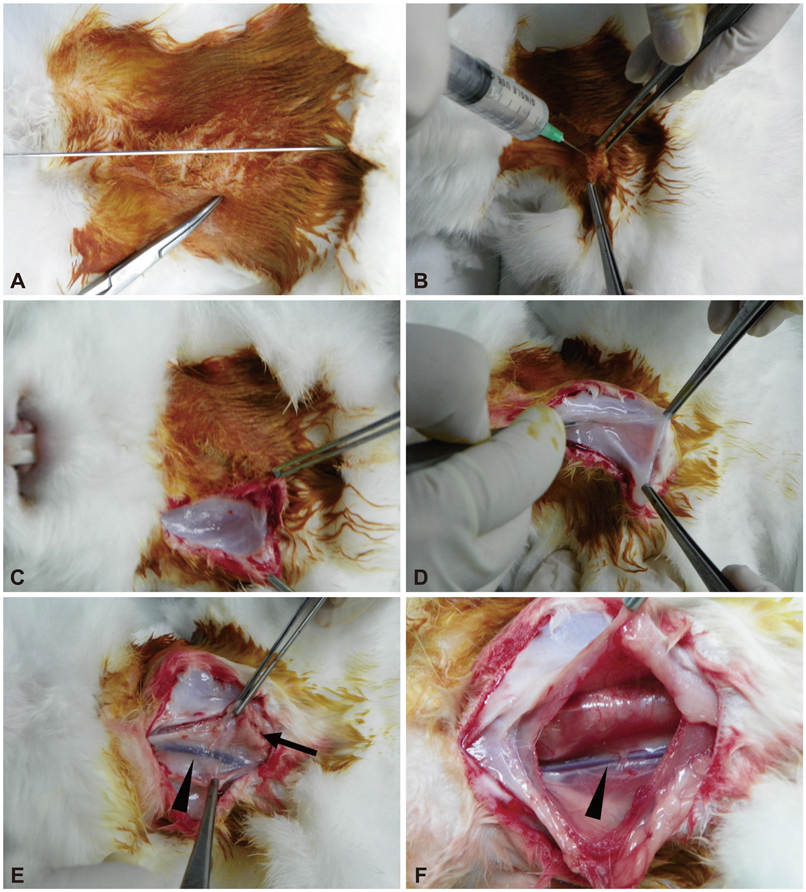
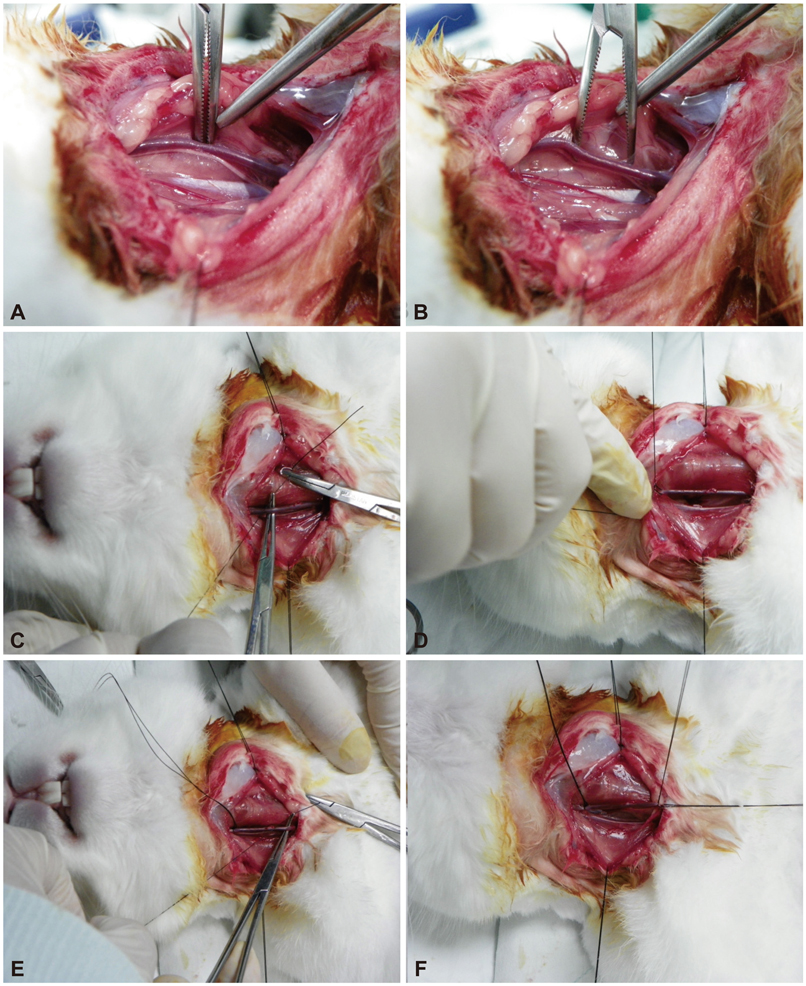
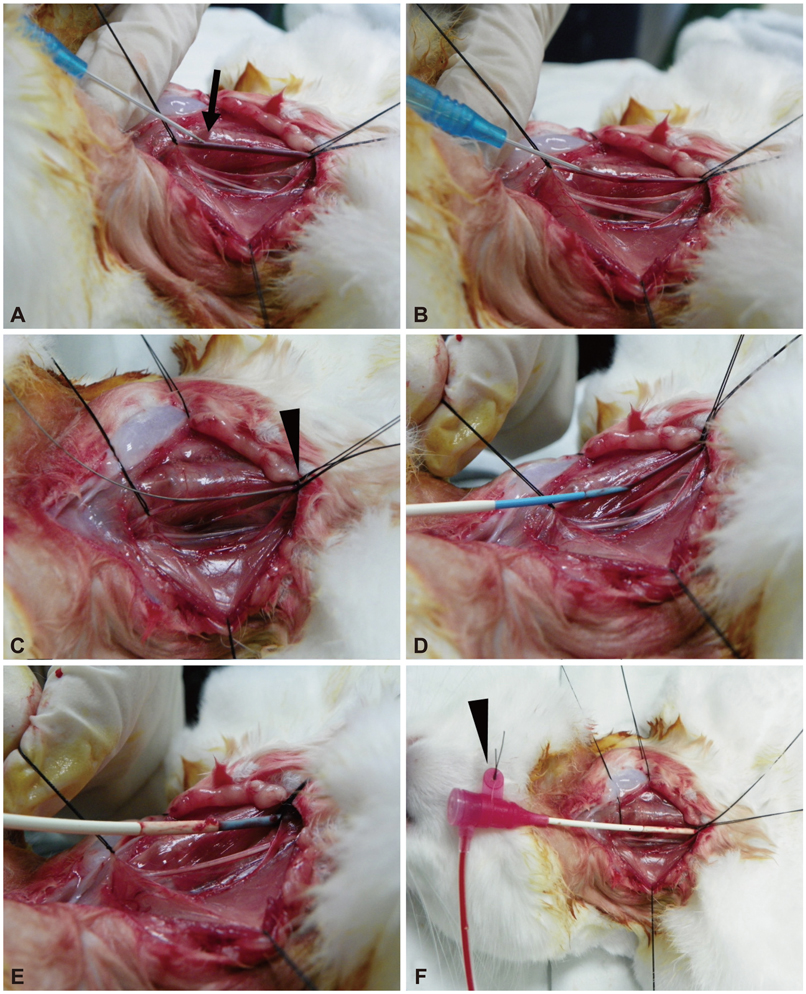
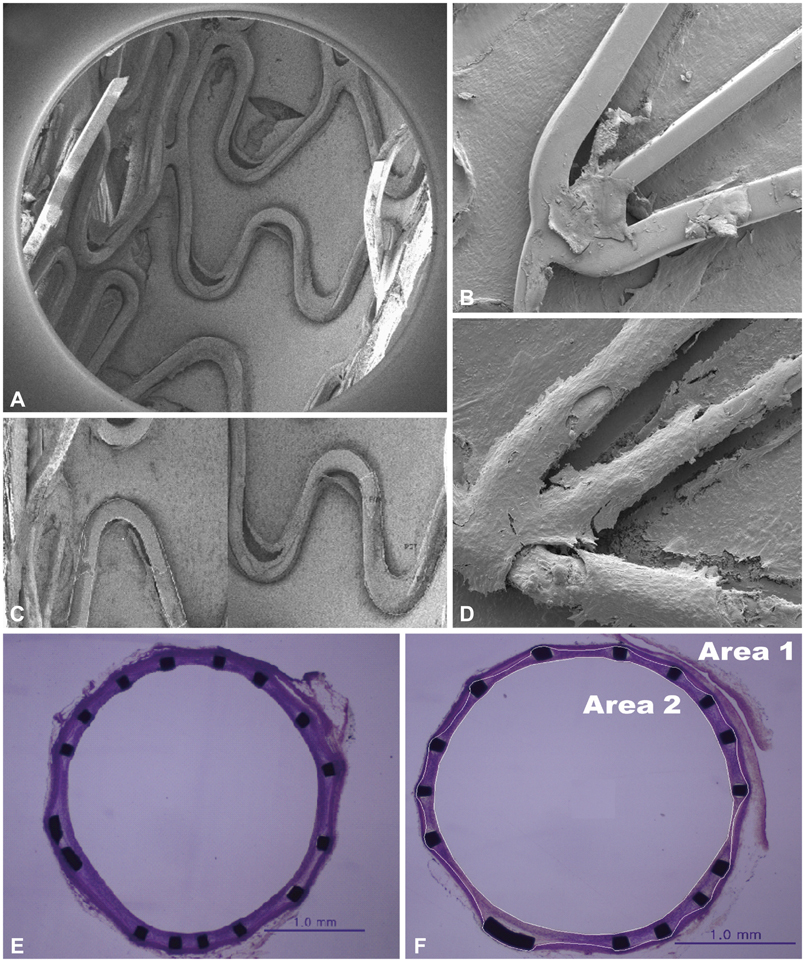
- Along with the development of innovative stent designs, preclinical trials in animal models are essential. Many animal models have been used and appear to yield comparable results to clinical trials despite substantial criticisms about their validity. Among the animal models, porcine coronary artery models have been the standard models for the preclinical evaluation of endovascular devices. However, rapid growth rate, high body weight potential, and the propensity to develop granulomatous inflammatory reactions are major limitations of the porcine coronary artery model. Compared with porcine coronary artery models, the comparative rabbit iliac artery model has the advantages of being small and easy to handle and relatively inexpensive. Furthermore, the rabbit model has been known to reliably reflect human restenosis histopathologically and have major advantages such as pairwise comparison, which makes each animal serve as its own control subject, therefore, maximizing its statistical power for comparative testing. However, despite the widespread use of this model, a systematic description of the procedure and harvest protocols has never been published. This article describes the surgical procedure, stent implantation procedure, method for tissue harvesting, and how measurements are performed. Although the results of animal models may not perfectly extrapolate to humans, the comparative rabbit iliac artery model may be a useful tool for assessing and comparing the efficacy of new coronary stents with conventional stent systems. This thorough description of the techniques required for vascular access, stent implantation, tissue preparation, and measurement, should aid investigators wishing to begin using the comparative rabbit iliac artery model.
Keyword
MeSH Terms
Figure
Reference
-
1. Simsek C, Serruys PW. Developments in coronary artery stenting: primum non nocere. Panminerva Med. 2011; 53:19–30.2. Fischman DL, Leon MB, Baim DS, et al. Stent Restenosis Study Investigators. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. N Engl J Med. 1994; 331:496–501.3. Serruys PW, van Hout B, Bonnier H, et al. Randomised comparison of implantation of heparin-coated stents with balloon angioplasty in selected patients with coronary artery disease (benestent II). Lancet. 1998; 352:673–681.4. Cook S, Ladich E, Nakazawa G, et al. Correlation of intravascular ultrasound findings with histopathological analysis of thrombus aspirates in patients with very late drug-eluting stent thrombosis. Circulation. 2009; 120:391–399.5. Finn AV, Kolodgie FD, Harnek J, et al. Differential response of delayed healing and persistent inflammation at sites of overlapping sirolimusor paclitaxel-eluting stents. Circulation. 2005; 112:270–278.6. Mauri L, Hsieh WH, Massaro JM, Ho KK, D'Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007; 356:1020–1029.7. Rogers C, Edelman ER. Endovascular stent design dictates experimental restenosis and thrombosis. Circulation. 1995; 91:2995–3001.8. Carter AJ, Aggarwal M, Kopia GA, et al. Long-term effects of polymer-based, slow-release, sirolimus-eluting stents in a porcine coronary model. Cardiovasc Res. 2004; 63:617–624.9. Serruys PW, Kutryk MJ, Ong AT. Coronary-artery stents. N Engl J Med. 2006; 354:483–495.10. Akin I, Schneider H, Ince H, et al. Second- and third-generation drug-eluting coronary stents: progress and safety. Herz. 2011; 36:190–196.11. Garg S, Serruys PW. Coronary stents: looking forward. J Am Coll Cardiol. 2010; 56:10 suppl. S43–S78.12. Touchard AG, Schwartz RS. Preclinical restenosis models: challenges and successes. Toxicol Pathol. 2006; 34:11–18.13. Robert R, Rioufol G, Finet G, et al. Experimental assessment of new stent technologies: validation of a comparative paired rabbit iliac artery study model. J Biomed Mater Res B Appl Biomater. 2004; 70:303–310.14. Schwartz RS, Chronos NA, Virmani R. Preclinical restenosis models and drug-eluting stents: still important, still much to learn. J Am Coll Cardiol. 2004; 44:1373–1385.15. Nakamura T, Winsor-Hines D, Yin X, et al. Vasomotor function and re-endothelialisation after implantation of biodegradable abluminal polymer coated paclitaxel-eluting stents in rabbit iliac arteries: a time-course study. EuroIntervention. 2012; 8:493–500.16. Nakazawa G, Nakano M, Otsuka F, et al. Evaluation of polymer-based comparator drug-eluting stents using a rabbit model of iliac artery atherosclerosis. Circ Cardiovasc Interv. 2011; 4:38–46.17. Doerning BJ, Brammer DW, Chrisp CE, Rush HG. Nephrotoxicity of tiletamine in New Zealand white rabbits. Lab Anim Sci. 1992; 42:267–269.18. Cho HJ, Kim HS, Lee MM, et al. Mobilized endothelial progenitor cells by granulocyte-macrophage colony-stimulating factor accelerate reendothelialization and reduce vascular inflammation after intravascular radiation. Circulation. 2003; 108:2918–2925.19. Van Dyck CJ, Hoymans VY, Bult H, et al. Resolute and Xience V polymer-based drug-eluting stents compared in an atherosclerotic rabbit double injury model. Catheter Cardiovasc Interv. 2013; 81:E259–E268.20. Sommer CM, Grenacher L, Stampfl U, et al. Impact of stent design on in-stent stenosis in a rabbit iliac artery model. Cardiovasc Intervent Radiol. 2010; 33:565–575.21. Robertson SA, Eberhart S. Efficacy of the intranasal route for administration of anesthetic agents to adult rabbits. Lab Anim Sci. 1994; 44:159–165.22. Che HL, Bae IH, Lim KS, et al. Suppression of post-angioplasty restenosis with an Akt1 siRNA-embedded coronary stent in a rabbit model. Biomaterials. 2012; 33:8548–8556.23. Lim WH, Seo WW, Choe W, et al. Stent coated with antibody against vascular endothelial-cadherin captures endothelial progenitor cells, accelerates re-endothelialization, and reduces neointimal formation. Arterioscler Thromb Vasc Biol. 2011; 31:2798–2805.24. Lee JM, Choe W, Kim BK, et al. Comparison of endothelialization and neointimal formation with stents coated with antibodies against CD34 and vascular endothelial-cadherin. Biomaterials. 2012; 33:8917–8927.25. Van Belle E, Tio FO, Couffinhal T, Maillard L, Passeri J, Isner JM. Stent endothelialization. Time course, impact of local catheter delivery, feasibility of recombinant protein administration, and response to cytokine expedition. Circulation. 1997; 95:438–448.26. Bayes-Genis A, Kantor B, Keelan PC, et al. Restenosis and hyperplasia: Animal Models. Curr Interv Cardiol Rep. 2000; 2:303–308.27. Perkins LE. Preclinical models of restenosis and their application in the evaluation of drug-eluting stent systems. Vet Pathol. 2010; 47:58–76.28. Asada Y, Kisanuki A, Tsuneyoshi A, Marutsuka K, Hatakeyama K, Sumiyoshi A. Effects of inflation pressure of balloon catheter on vascular injuries and subsequent development of intimal hyperplasia in rabbit aorta. Atherosclerosis. 1996; 121:45–53.29. Lowe HC, Schwartz RS, Mac Neill BD, et al. The porcine coronary model of in-stent restenosis: current status in the era of drug-eluting stents. Catheter Cardiovasc Interv. 2003; 60:515–523.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The preventive effects of the heparin-coated coronary stent in a porcine coronary stent restenosis model
- Comparison of Core(R) stent and Palmaz-Schatz(R) stent in a Porcine Stent Restenosis Model
- Preclinical Evaluation of a Novel Polymer-free Everolimus-eluting Stent in a Mid-term Porcine Coronary Restenosis Model
- Coronary Stent on Coronary CT Angiography: Assessment with Model-Based Iterative Reconstruction Technique
- Coronary Stent Fracture in a Patient with an Atrial Septal Defect and Severe Pulmonary Hypertension