Ann Lab Med.
2017 Mar;37(2):147-150. 10.3343/alm.2017.37.2.147.
Performance of the Real-Q EBV Quantification Kit for Epstein-Barr Virus DNA Quantification in Whole Blood
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Samsung Biomedical Research Institute, Samsung Medical Center, Seoul, Korea. changski@skku.edu
- 2Center for Clinical Medicine, Samsung Biomedical Research Institute, Samsung Medical Center, Seoul, Korea.
- KMID: 2373632
- DOI: http://doi.org/10.3343/alm.2017.37.2.147
Abstract
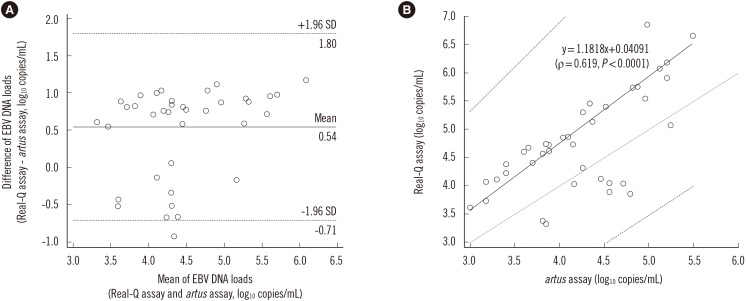
- There has been increasing interest in standardized and quantitative Epstein-Barr virus (EBV) DNA testing for the management of EBV disease. We evaluated the performance of the Real-Q EBV Quantification Kit (BioSewoom, Korea) in whole blood (WB). Nucleic acid extraction and real-time PCR were performed by using the MagNA Pure 96 (Roche Diagnostics, Germany) and 7500 Fast real-time PCR system (Applied Biosystems, USA), respectively. Assay sensitivity, linearity, and conversion factor were determined by using the World Health Organization international standard diluted in EBV-negative WB. We used 81 WB clinical specimens to compare performance of the Real-Q EBV Quantification Kit and artus EBV RG PCR Kit (Qiagen, Germany). The limit of detection (LOD) and limit of quantification (LOQ) for the Real-Q kit were 453 and 750 IU/mL, respectively. The conversion factor from EBV genomic copies to IU was 0.62. The linear range of the assay was from 750 to 10ⶠIU/mL. Viral load values measured with the Real-Q assay were on average 0.54 logâ‚â‚€ copies/mL higher than those measured with the artus assay. The Real-Q assay offered good analytical performance for EBV DNA quantification in WB.
MeSH Terms
Figure
Reference
-
1. Gulley ML, Tang W. Using Epstein-Barr viral load assays to diagnose, monitor, and prevent posttransplant lymphoproliferative disorder. Clin Microbiol Rev. 2010; 23:350–366. PMID: 20375356.2. Heslop HE. How I treat EBV lymphoproliferation. Blood. 2009; 114:4002–4008. PMID: 19724053.3. San-Juan R, Comoli P, Caillard S, Moulin B, Hirsch HH, Meylan P. Epstein-Barr virus-related post-transplant lymphoproliferative disorder in solid organ transplant recipients. Clin Microbiol Infect. 2014; 20(S7):109–118. PMID: 24475976.4. Gärtner B, Preiksaitis JK. EBV viral load detection in clinical virology. J Clin Virol. 2010; 48:82–90. PMID: 20395167.5. Hayden RT, Yan X, Wick MT, Rodriguez AB, Xiong X, Ginocchio CC, et al. Factors contributing to variability of quantitative viral PCR results in proficiency testing samples: a multivariate analysis. J Clin Microbiol. 2012; 50:337–345. PMID: 22116152.6. Fryer JF, Heath AB, Wilkinson DE, Minor PD. Collaborative study to evaluate the proposed 1st WHO international standard for Epstein-Barr Virus (EBV) for nucleic acid amplification (NAT)-based assays. Geneva, Switzerland: World Health Organization;2011.7. Smith TF, Espy MJ, Mandrekar J, Jones MF, Cockerill FR, Patel R. Quantitative real-time polymerase chain reaction for evaluating DNAemia due to cytomegalovirus, Epstein-Barr virus, and BK virus in solid-organ transplant recipients. Clin Infect Dis. 2007; 45:1056–1061. PMID: 17879925.8. Ha J, Park Y, Shim J, Kim HS. Evaluation of real-time PCR kits for Epstein-Barr virus DNA assays. Lab Med Online. 2016; 6:31–35.9. CLSI. Evaluation of detection capability for clinical laboratory measurement procedures; Approved Guideline – Second ed. CLSI document EP17-A2. Wayne, PA: Clinical and Laboratory Standards Institute;2012.10. Gullett JC, Nolte FS. Quantitative nucleic acid amplification methods for viral infections. Clin Chem. 2015; 61:72–78. PMID: 25403817.11. Semenova T, Lupo J, Alain S, Perrin-Confort G, Grossi L, Dimier J, et al. Multicenter evaluation of whole-blood Epstein-Barr viral load standardization using the WHO international standard. J Clin Microbiol. 2016; 54:1746–1750. PMID: 27076661.12. Ruf S, Behnke-Hall K, Gruhn B, Bauer J, Horn M, Beck J, et al. Comparison of six different specimen types for Epstein-Barr viral load quantification in peripheral blood of pediatric patients after heart transplantation or after allogeneic hematopoietic stem cell transplantation. J Clin Virol. 2012; 53:186–194. PMID: 22182950.13. Ouedraogo DE, Bollore K, Viljoen J, Foulongne V, Reynes J, Cartron G, et al. Comparison of EBV DNA viral load in whole blood, plasma, B-cells and B-cell culture supernatant. J Med Virol. 2014; 86:851–856. PMID: 24265067.14. Germi R, Lupo J, Semenova T, Larrat S, Magnat N, Grossi L, et al. Comparison of commercial extraction systems and PCR assays for quantification of Epstein-Barr virus DNA load in whole blood. J Clin Microbiol. 2012; 50:1384–1389. PMID: 22238432.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nucleic Acid Extraction for the Quantification of Cytomegalovirus and Epstein-Barr Virus
- Performance Evaluation of the ELITe InGenius System for Detecting Cytomegalovirus, EpsteinBarr Virus, and BK Virus Infections
- Evaluation of Real-time PCR Kits for Epstein-Barr Virus DNA Assays
- Diagnostic Performance and Comparative Evaluation of the Architect, Liaison, and Platelia Epstein-Barr Virus Antibody Assays
- Anticonvulsant Hypersensitivity Syndrome Associated with Epstein-Barr Virus Reactivation