Ann Lab Med.
2016 Nov;36(6):542-549. 10.3343/alm.2016.36.6.542.
Soluble ST2 Levels and Left Ventricular Structure and Function in Patients With Metabolic Syndrome
- Affiliations
-
- 1Faculty of Medicine, University of Belgrade, Belgrade, Serbia.
- 2University Hospital Centre "Dr DragisaMisovic-Dedinje", Belgrade, Serbia.
- 3Miguel Servet Foundation-Navarrabiomed, Pamplona, Spain.
- 4Complejo Hospitalario de Navarra, Pamplona, Spain.
- 5School of Medicine and Psychology, La Sapienza University, Sant'Andrea Hospital, Rome, Italy. salvatore.disomma@uniroma1.it
- KMID: 2373592
- DOI: http://doi.org/10.3343/alm.2016.36.6.542
Abstract
- BACKGROUND
A biomarker that is of great interest in relation to adverse cardiovascular events is soluble ST2 (sST2), a member of the interleukin family. Considering that metabolic syndrome (MetS) is accompanied by a proinflammatory state, we aimed to assess the relationship between sST2 and left ventricular (LV) structure and function in patients with MetS.
METHODS
A multicentric, cross-sectional study was conducted on180 MetS subjects with normal LV ejection fraction as determined by echocardiography. LV hypertrophy (LVH) was defined as an LV mass index greater than the gender-specific upper limit of normal as determined by echocardiography. LV diastolic dysfunction (DD) was assessed by pulse-wave and tissue Doppler imaging. sST2 was measured by using a quantitative monoclonal ELISA assay.
RESULTS
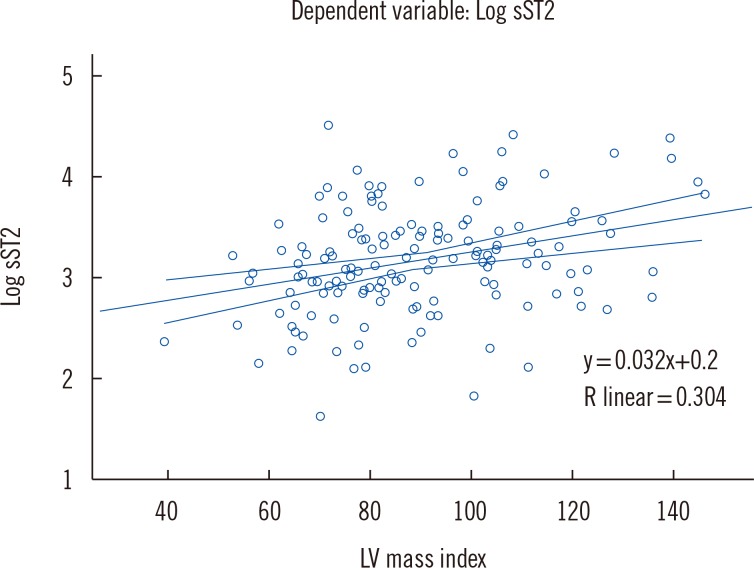
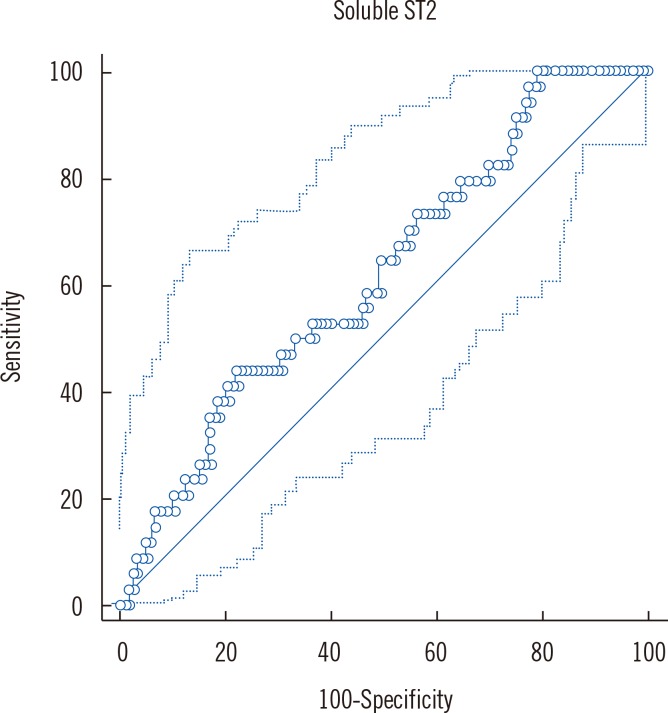
LV mass index (β=0.337, P<0.001, linear regression) was independently associated with sST2 concentrations. Increased sST2 was associated with an increased likelihood of LVH [Exp (B)=2.20, P=0.048, logistic regression] and increased systolic blood pressure [Exp (B)=1.02, P=0.05, logistic regression]. Comparing mean sST2 concentrations (adjusted for age, body mass index, gender) between different LV remodeling patterns, we found the greatest sST2 level in the group with concentric hypertrophy. There were no differences in sST2 concentration between groups with and without LV DD.
CONCLUSIONS
Increased sST2 concentration in patients with MetS was associated with a greater likelihood of exhibiting LVH. Our results suggest that inflammation could be one of the principal triggering mechanisms for LV remodeling in MetS.
MeSH Terms
-
Adult
Age Factors
Aged
Area Under Curve
Blood Pressure
Body Mass Index
Cross-Sectional Studies
Echocardiography, Doppler
Enzyme-Linked Immunosorbent Assay
Female
Humans
Hypertrophy, Left Ventricular/diagnostic imaging
Interleukin-1 Receptor-Like 1 Protein/*analysis
Linear Models
Logistic Models
Male
Metabolic Syndrome X/metabolism/*physiopathology
Middle Aged
ROC Curve
Sex Factors
Ventricular Function, Left/*physiology
Ventricular Remodeling/physiology
Interleukin-1 Receptor-Like 1 Protein
Figure
Reference
-
1. Alberti G, Zimmet P, Shaw J, Grundy SM. The IDF consensus worldwide definition of the metabolic syndrome. International Diabetes Federation;2006.2. Hunt KJ, ResendezRG , Williams K, Haffner SM, Stern MP. San Antonio Heart Study. National Cholesterol Education Program versus World Health Organization metabolic syndrome in relation to all-cause and cardiovascular mortality in the San Antonio Heart Study. Circulation. 2004; 110:1251–1257. PMID: 15326061.3. Kim SH. Metabolic syndrome and myocardial contractile reserve. J Cardiovasc Ultrasound. 2011; 19:174–175. PMID: 22259659.4. de las Fuentes L, Brown AL, Mathews SJ, Waggoner AD, Soto PF, Gropler RJ, et al. Metabolic syndrome is associated with abnormal left ventricular diastolic function independent of left ventricular mass. Eur Heart J. 2007; 28:553–559. PMID: 17311827.5. Tadic M, Ivanovic B, Kostic N, Simic D, Matic D, Celic V. Metabolic syndrome and left ventricular function: is the number of criteria actually important? Med Sci Monit. 2012; 18:CR282–CR289. PMID: 22534707.6. O'Neill L. The Toll/interleukin-1 receptor domain: a molecular switch for inflammation and host defence. Biochem Soc Trans. 2000; 28:557–563. PMID: 11044374.7. Shimpo M, Morrow DA, Weinberg EO, Sabatine MS, Murphy SA, Antman EM, et al. Serum levels of the interleukin-1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation. 2004; 109:2186–2190. PMID: 15117853.8. Rehman SU, Mueller T, Januzzi JL Jr. Characteristics of the novel interleukin family biomarker ST2 in patients with acute heart failure. J Am Coll Cardiol. 2008; 52:1458–1465. PMID: 19017513.9. Weinberg EO, Shimpo M, De Keulenaer GW, MacGillivray C, Tominaga S, Solomon SD, et al. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002; 106:2961–2966. PMID: 12460879.10. Weinberg EO, Shimpo M, Hurwitz S, Tominaga S, Rouleau JL, Lee RT. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation. 2003; 107:721–726. PMID: 12578875.11. Coglianese EE, Larson MG, Vasan RS, Ho JE, Ghorbani A, McCabe EL, et al. Distribution and clinical correlates of the interleukin receptor family member soluble ST2 in the Framingham Heart Study. Clin Chem. 2012; 58:1673–1681. PMID: 23065477.12. Ojji DB, Opie LH, Lecour S, Lacerda L, Adeyemi O, Sliwa K. Relationship between left ventricular geometry and soluble ST2 in a cohort of hypertensive patients. J Clin Hypertens (Greenwich). 2013; 15:899–904. PMID: 24168195.13. Zeyda M, Wernly B, Demyanets S, Kaun C, Hämmerle M, Hantusch B, et al. Severe obesity increases adipose tissue expression of interleukin-33 and its receptor ST2, both predominantly detectable in endothelial cells of human adipose tissue. Int J Obes (Lond). 2013; 37:658–665. PMID: 22828942.14. Fousteris E, Melidonis A, Panoutsopoulos G, Tzirogiannis K, Foussas S, Theodosis-Georgilas A, et al. Toll/interleukin-1 receptor member ST2 exhibits higher soluble levels in type 2 diabetes, especially when accompanied with left ventricular diastolic dysfunction. Cardiovasc Diabetol. 2011; 10:101. PMID: 22104207.15. Chen LQ, de Lemos JA, Das SR, Ayers CR, Rohatgi A. Soluble ST2 is associated with all-cause and cardiovascular mortality in a population-based cohort: the Dallas Heart Study. Clin Chem. 2013; 59:536–546. PMID: 23220272.16. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013; 34:2159–2219. PMID: 23771844.17. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome:ajoint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009; 120:1640–1645. PMID: 19805654.18. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome-a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006; 23:469–480. PMID: 16681555.19. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015; 28:1–39. PMID: 25559473.20. Quiñones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002; 15:167–184. PMID: 11836492.21. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009; 10:165–193. PMID: 19270053.22. Oh JK, Park SJ, Nagueh SF. Established and novel clinical applications of diastolic function assessment by echocardiography. Circ Cardiovasc Imaging. 2011; 4:444–455. PMID: 21772012.23. Westcott DJ, DelpropostoJB , Geletka LM, Wang T, Singer K, Saltiel AR, et al. MGL1 promotes adipose tissue inflammation and insulin resistance by regulating 7/4hi monocytes in obesity. J Exp Med. 2009; 206:3143–3156. PMID: 19995956.24. Ciccone MM, Cortese F, Gesualdo M, Riccardi R, Di Nunzio D, Moncelli M, et al. A novel cardiac bio-marker: ST2: areview. Molecules. 2013; 18:15314–15328. PMID: 24335613.25. Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013; 39:1003–1018. PMID: 24332029.26. Demyanets S, Kaun C, Pentz R, Krychtiuk KA, Rauscher S, Pfaffenberger S, et al. Components of the interleukin-33/ST2 system are differentially expressed and regulated in human cardiac cells and in cells of the cardiac vasculature. J Mol Cell Cardiol. 2013; 60:16–26. PMID: 23567618.27. Wang YC, Yu CC, Chiu FC, Tsai CT, Lai LP, Hwang JJ, et al. Soluble ST2 as a biomarker for detecting stable heart failure with a normal ejection fraction in hypertensive patients. J Card Fail. 2013; 19:163–168. PMID: 23482076.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Elevated levels of soluble ST2 but not galectin-3 are associated with increased risk of mortality in hemodialysis patients
- Correlations between Serum Inflammation Factors and Left Ventricular Remodeling in Acute ST Segment Elevation Myocardial Infarction
- A Study of Left Ventricular Function by Digitized Echocardiograms in Dilated Cardiomyopathy
- Three Cases of Stress Induced Transient LV Dysfunction: Stress Induced Cardiomyopathy
- Are Soluble ST2 Levels in the Picogram or Nanogram Range in Serum of Healthy Subjects/Disease Patients?