J Gastric Cancer.
2016 Mar;16(1):21-27. 10.5230/jgc.2016.16.1.21.
Prognostic and Clinicopathological Significance of Transducer-Like Enhancer of Split 1 Expression in Gastric Cancer
- Affiliations
-
- 1Department of Pathology, Soonchunhyang University Cheonan Hospital, Cheonan, Korea.
- 2Department of General Surgery, Soonchunhyang University Cheonan Hospital, Cheonan, Korea. msslee@schmc.ac.kr
- 3Department of Pathology, Soonchunhyang University College of Medicine, Cheonan, Korea.
- KMID: 2372549
- DOI: http://doi.org/10.5230/jgc.2016.16.1.21
Abstract
- PURPOSE
Transducer-like enhancer of split 1 (TLE1) is a member of the Groucho/TLE family of transcriptional co-repressors that regulate the transcriptional activity of numerous genes. TLE1 is involved in the tumorigenesis of various tumors. We investigated the prognostic significance of TLE1 expression and its association with clinicopathological parameters in gastric cancer (GC) patients.
MATERIALS AND METHODS
Immunohistochemical analysis of six tissue microarrays was performed to examine TLE1 expression using 291 surgically resected GC specimens from the Soonchunhyang University Cheonan Hospital between July 2006 and December 2009.
RESULTS
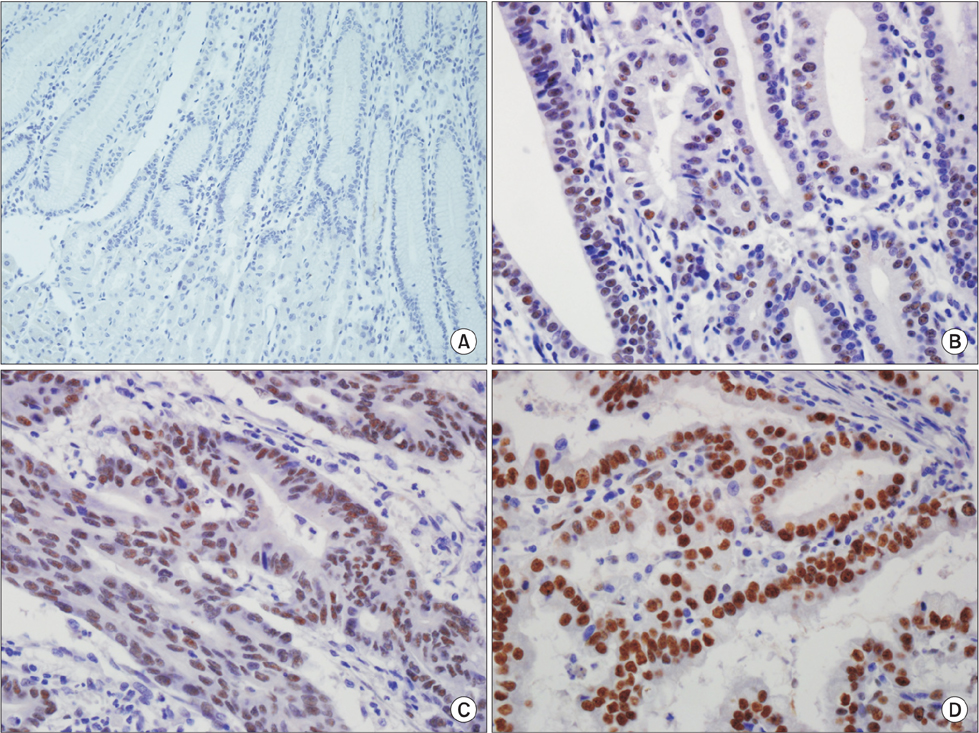
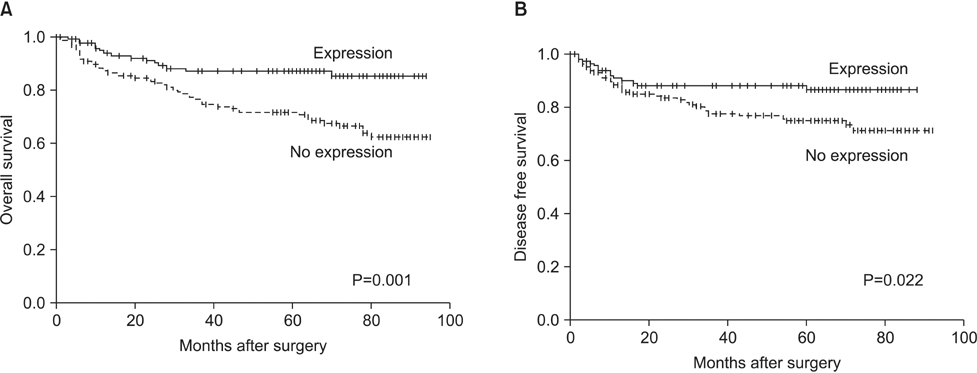
In the non-neoplastic gastric mucosa, TLE1 expression was negative. In GC, 121 patients (41.6%) were positive for TLE1. The expression of TLE1 was significantly associated with male gender (P=0.021), less frequent lymphatic (P=0.017) or perineural invasion (P=0.029), intestinal type according to the Lauren classification (P=0.024), good histologic grade (P<0.001), early pathologic T-stage (P=0.012), and early American Joint Committee on Cancer stage (P=0.022). In the Kaplan-Meier analysis, the TLE1 expression was significantly associated with longer disease-free (P=0.022) and overall (P=0.001) survival rates.
CONCLUSIONS
We suggested that TLE1 expression is a good prognostic indicator in GCs.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Clinicopathologic and Prognostic Significance of Transducin-Like Enhancer of Split 1 Protein Expression in Invasive Breast Cancer
Ji-Hye Lee, Sang Byung Bae, Mee-Hye Oh, Hyun Deuk Cho, Si-Hyong Jang, Soon Auck Hong, Junhun Cho, Sung Yong Kim, Sun Wook Han, Jong Eun Lee, Han Jo Kim, Hyun Ju Lee
J Breast Cancer. 2017;20(1):45-53. doi: 10.4048/jbc.2017.20.1.45.
Reference
-
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.2. Chen G, Courey AJ. Groucho/TLE family proteins and transcriptional repression. Gene. 2000; 249:1–16.3. Arce L, Pate KT, Waterman ML. Groucho binds two conserved regions of LEF-1 for HDAC-dependent repression. BMC Cancer. 2009; 9:159.4. Ghosh HS, Spencer JV, Ng B, McBurney MW, Robbins PD. Sirt1 interacts with transducin-like enhancer of split-1 to inhibit nuclear factor kappaB-mediated transcription. Biochem J. 2007; 408:105–111.5. Buscarlet M, Hermann R, Lo R, Tang Y, Joachim K, Stifani S. Cofactor-activated phosphorylation is required for inhibition of cortical neuron differentiation by Groucho/TLE1. PLoS One. 2009; 4:e8107.6. Flowers EB, Poole RJ, Tursun B, Bashllari E, Pe'er I, Hobert O. The Groucho ortholog UNC-37 interacts with the short Groucho-like protein LSY-22 to control developmental decisions in C. elegans. Development. 2010; 137:1799–1805.7. Allander SV, Illei PB, Chen Y, Antonescu CR, Bittner M, Ladanyi M, et al. Expression profiling of synovial sarcoma by cDNA microarrays: association of ERBB2, IGFBP2, and ELF3 with epithelial differentiation. Am J Pathol. 2002; 161:1587–1595.8. Nagayama S, Katagiri T, Tsunoda T, Hosaka T, Nakashima Y, Araki N, et al. Genome-wide analysis of gene expression in synovial sarcomas using a cDNA microarray. Cancer Res. 2002; 62:5859–5866.9. Jagdis A, Rubin BP, Tubbs RR, Pacheco M, Nielsen TO. Prospective evaluation of TLE1 as a diagnostic immunohistochemical marker in synovial sarcoma. Am J Surg Pathol. 2009; 33:1743–1751.10. Foo WC, Cruise MW, Wick MR, Hornick JL. Immunohistochemical staining for TLE1 distinguishes synovial sarcoma from histologic mimics. Am J Clin Pathol. 2011; 135:839–844.11. Kosemehmetoglu K, Vrana JA, Folpe AL. TLE1 expression is not specific for synovial sarcoma: a whole section study of 163 soft tissue and bone neoplasms. Mod Pathol. 2009; 22:872–878.12. Terry J, Saito T, Subramanian S, Ruttan C, Antonescu CR, Goldblum JR, et al. TLE1 as a diagnostic immunohistochemical marker for synovial sarcoma emerging from gene expression profiling studies. Am J Surg Pathol. 2007; 31:240–246.13. Matsuyama A, Hisaoka M, Iwasaki M, Iwashita M, Hisanaga S, Hashimoto H. TLE1 expression in malignant mesothelioma. Virchows Arch. 2010; 457:577–583.14. Fraga MF, Berdasco M, Ballestar E, Ropero S, Lopez-Nieva P, Lopez-Serra L, et al. Epigenetic inactivation of the Groucho homologue gene TLE1 in hematologic malignancies. Cancer Res. 2008; 68:4116–4122.15. Dayyani F, Wang J, Yeh JR, Ahn EY, Tobey E, Zhang DE, et al. Loss of TLE1 and TLE4 from the del(9q) commonly deleted region in AML cooperates with AML1-ETO to affect myeloid cell proliferation and survival. Blood. 2008; 111:4338–4347.16. Zhang L, Yang L, Liu X, Chen W, Chang L, Chen L, et al. MicroRNA-657 promotes tumorigenesis in hepatocellular carcinoma by targeting transducin-like enhancer protein 1 through nuclear factor kappa B pathways. Hepatology. 2013; 57:1919–1930.17. Brunquell C, Biliran H, Jennings S, Ireland SK, Chen R, Ruoslahti E. TLE1 is an anoikis regulator and is downregulated by Bit1 in breast cancer cells. Mol Cancer Res. 2012; 10:1482–1495.18. Yao X, Ireland SK, Pham T, Temple B, Chen R, Raj MH, et al. TLE1 promotes EMT in A549 lung cancer cells through suppression of E-cadherin. Biochem Biophys Res Commun. 2014; 455:277–284.19. Lee HW, Kim EH, Oh MH. Clinicopathologic implication of ezrin expression in non-small cell lung cancer. Korean J Pathol. 2012; 46:470–477.20. Gasperowicz M, Otto F. Mammalian Groucho homologs: redundancy or specificity? J Cell Biochem. 2005; 95:670–687.21. Jan Y, Matter M, Pai JT, Chen YL, Pilch J, Komatsu M, et al. A mitochondrial protein, Bit1, mediates apoptosis regulated by integrins and Groucho/TLE corepressors. Cell. 2004; 116:751–762.22. Allen T, van Tuyl M, Iyengar P, Jothy S, Post M, Tsao MS, et al. Grg1 acts as a lung-specific oncogene in a transgenic mouse model. Cancer Res. 2006; 66:1294–1301.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinicopathological Significance of p53 and HSP27 in Gastric-cancer Patients
- Prognostic Value of p53 and Proliferating Cell Nuclear Antigen ( PCNA ) in Stage 3 Gastric Carcinoma
- Clinicopathological Features and Survival of Patients with Gastric Cancer with a Family History: a Large Analysis of 2,736 Patients with Gastric Cancer
- Clinicopathologic and Prognostic Significance of Transducin-Like Enhancer of Split 1 Protein Expression in Invasive Breast Cancer
- Clinicopathological Features of Borrmann Type IV Gastric Carcinomas