J Gastric Cancer.
2013 Dec;13(4):214-225.
Test Execution Variation in Peritoneal Lavage Cytology Could Be Related to Poor Diagnostic Accuracy and Stage Migration in Patients with Gastric Cancer
- Affiliations
-
- 1Department of Surgery, Korea Cancer Center Hospital, Korea Institute of Radiological & Medical Sciences, Seoul, Korea. shjin@kcch.re.kr
- 2Department of Surgery, Dongnam Institute of Radiological & Medical Sciences, Busan, Korea.
- 3Department of Pathology, Korea Cancer Center Hospital, Korea Institute of Radiological & Medical Sciences, Seoul, Korea.
- 4Department of Surgery, Konkuk University School of Medicine, Seoul, Korea.
Abstract
- PURPOSE
Peritoneal lavage cytology is part of the routine staging workup for patients with advanced gastric cancer. However, no quality assurance study has been conducted to show variations or biases in peritoneal lavage cytology results. The aim of this study was to demonstrate a test execution variation in peritoneal lavage cytology between investigating surgeons.
MATERIALS AND METHODS
A prospective cohort study was designed for determination of the positive rate of peritoneal lavage cytology using a liquid-based preparation method in patients with potentially curable advanced gastric cancer (cT2~4/N0~2/M0). One hundred thirty patients were enrolled and underwent laparotomy, peritoneal lavage cytology, and standard gastrectomy, which were performed by 3 investigating surgeons. Data were analyzed using the chi-square test and a logistic regression model.
RESULTS
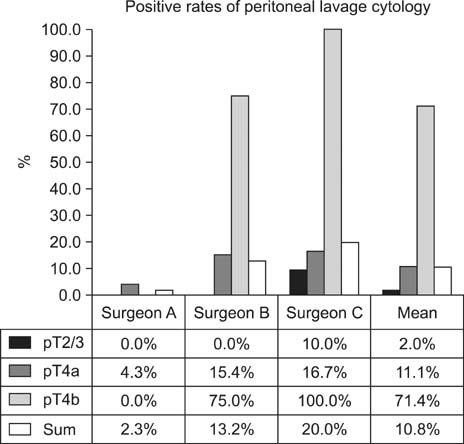
The overall positive peritoneal cytology rate was 10.0%. Subgroup positive rates were 5.3% in pT1 cancer, 2.0% in pT2/3 cancer, 11.1% in pT4a cancer, and 71.4% in pT4b cancer. In univariate analysis, positive peritoneal cytology showed significant correlation with pT stage, lymphatic invasion, vascular invasion, ascites, and the investigating surgeon. We found the positive rate to be 2.1% for surgeon A, 10.2% for surgeon B, and 20.6% for surgeon C (P=0.024). Multivariate analysis identified pT stage, ascites, and the investigating surgeon to be significant risk factors for positive peritoneal cytology.
CONCLUSIONS
The peritoneal lavage cytology results were significantly affected by the investigating surgeon, providing strong evidence of test execution variation that could be related to poor diagnostic accuracy and stage migration in patients with advanced gastric cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Ikeguchi M, Oka A, Tsujitani S, Maeta M, Kaibara N. Relationship between area of serosal invasion and intraperitoneal free cancer cells in patients with gastric cancer. Anticancer Res. 1994; 14:2131–2134.2. Bando E, Yonemura Y, Takeshita Y, Taniguchi K, Yasui T, Yoshimitsu Y, et al. Intraoperative lavage for cytological examination in 1,297 patients with gastric carcinoma. Am J Surg. 1999; 178:256–262.
Article3. Suzuki T, Ochiai T, Hayashi H, Hori S, Shimada H, Isono K. Peritoneal lavage cytology findings as prognostic factor for gastric cancer. Semin Surg Oncol. 1999; 17:103–107.
Article4. Nakajima T, Harashima S, Hirata M, Kajitani T. Prognostic and therapeutic values of peritoneal cytology in gastric cancer. Acta Cytol. 1978; 22:225–229.5. Iitsuka Y, Kaneshima S, Tanida O, Takeuchi T, Koga S. Intraperitoneal free cancer cells and their viability in gastric cancer. Cancer. 1979; 44:1476–1480.
Article6. Funami Y, Tokumoto N, Miyauchi H, Ochiai T, Kuga K. Prognostic value of peritoneal lavage cytology and chemotherapy during surgery for advanced gastric cancer. Int Surg. 1999; 84:220–224.7. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma - 2nd English edition -. Gastric Cancer. 1998; 1:10–24.8. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer;2010.9. Shimada S, Tanaka E, Marutsuka T, Honmyo U, Tokunaga H, Yagi Y, et al. Extensive intraoperative peritoneal lavage and chemotherapy for gastric cancer patients with peritoneal free cancer cells. Gastric Cancer. 2002; 5:168–172.
Article10. Yonemura Y, Bandou E, Sawa T, Yoshimitsu Y, Endou Y, Sasaki T, et al. Neoadjuvant treatment of gastric cancer with peritoneal dissemination. Eur J Surg Oncol. 2006; 32:661–665.
Article11. Nakagawa S, Nashimoto A, Yabusaki H. Role of staging laparoscopy with peritoneal lavage cytology in the treatment of locally advanced gastric cancer. Gastric Cancer. 2007; 10:29–34.
Article12. Kuramoto M, Shimada S, Ikeshima S, Matsuo A, Yagi Y, Matsuda M, et al. Extensive intraoperative peritoneal lavage as a standard prophylactic strategy for peritoneal recurrence in patients with gastric carcinoma. Ann Surg. 2009; 250:242–246.
Article13. Fujiwara Y, Nishida T, Takiguchi S, Nakajima K, Miyata H, Yamasaki M, et al. Feasibility study of S-1 and intraperitoneal docetaxel combination chemotherapy for gastric cancer with peritoneal dissemination. Anticancer Res. 2010; 30:1335–1339.14. Ishigami H, Kitayama J, Kaisaki S, Hidemura A, Kato M, Otani K, et al. Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol. 2010; 21:67–70.
Article15. Lorenzen S, Panzram B, Rosenberg R, Nekarda H, Becker K, Schenk U, et al. Prognostic significance of free peritoneal tumor cells in the peritoneal cavity before and after neoadjuvant chemotherapy in patients with gastric carcinoma undergoing potentially curative resection. Ann Surg Oncol. 2010; 17:2733–2739.
Article16. Mezhir JJ, Shah MA, Jacks LM, Brennan MF, Coit DG, Strong VE. Positive peritoneal cytology in patients with gastric cancer: natural history and outcome of 291 patients. Ann Surg Oncol. 2010; 17:3173–3180.
Article17. Greenhalgh T. How to read a paper. Papers that report diagnostic or screening tests. BMJ. 1997; 315:540–543.
Article18. Hutchinson ML, Zahniser DJ, Sherman ME, Herrero R, Alfaro M, Bratti MC, et al. Utility of liquid-based cytology for cervical carcinoma screening: results of a population-based study conducted in a region of Costa Rica with a high incidence of cervical carcinoma. Cancer. 1999; 87:48–55.
Article19. Abulafia O, Pezzullo JC, Sherer DM. Performance of ThinPrep liquid-based cervical cytology in comparison with conventionally prepared Papanicolaou smears: a quantitative survey. Gynecol Oncol. 2003; 90:137–144.
Article20. Davey E, Barratt A, Irwig L, Chan SF, Macaskill P, Mannes P, et al. Effect of study design and quality on unsatisfactory rates, cytology classifications, and accuracy in liquid-based versus conventional cervical cytology: a systematic review. Lancet. 2006; 367:122–132.
Article21. Hawkins DM, Garrett JA, Stephenson B. Some issues in resolution of diagnostic tests using an imperfect gold standard. Stat Med. 2001; 20:1987–2001.
Article22. Whiting P, Rutjes AW, Reitsma JB, Glas AS, Bossuyt PM, Kleijnen J. Sources of variation and bias in studies of diagnostic accuracy: a systematic review. Ann Intern Med. 2004; 140:189–202.
Article23. Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005; 37:360–363.24. Dodd LG, Sneige N, Villarreal Y, Fanning CV, Staerkel GA, Caraway NP, et al. Quality-assurance study of simultaneously sampled, non-correlating cervical cytology and biopsies. Diagn Cytopathol. 1993; 9:138–144.
Article25. Tritz DM, Weeks JA, Spires SE, Sattich M, Banks H, Cibull ML, et al. Etiologies for non-correlating cervical cytologies and biopsies. Am J Clin Pathol. 1995; 103:594–597.
Article26. Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002; 97:2614–2618.
Article27. Cholongitas E, Senzolo M, Standish R, Marelli L, Quaglia A, Patch D, et al. A systematic review of the quality of liver biopsy specimens. Am J Clin Pathol. 2006; 125:710–721.
Article28. Corkill M, Knapp D, Martin J, Hutchinson ML. Specimen adequacy of ThinPrep sample preparations in a direct-to-vial study. Acta Cytol. 1997; 41:39–44.
Article29. Papanicolaou GN. Cytologic diagnosis of uterine cancer by examination of vaginal and uterine secretions. Am J Clin Pathol. 1949; 19:301–308.
Article30. Marshall PN. Papanicolaou staining--a review. Microsc Acta. 1983; 87:233–243.31. Jass JR, Smith M. Sialic acid and epithelial differentiation in colorectal polyps and cancer--a morphological, mucin and lectin histochemical study. Pathology. 1992; 24:233–242.
Article32. Chuwa EW, Khin LW, Chan WH, Ong HS, Wong WK. Prognostic significance of peritoneal lavage cytology in gastric cancer in Singapore. Gastric Cancer. 2005; 8:228–237.
Article33. Yamamoto M, Matsuyama A, Kameyama T, Okamoto M, Okazaki J, Utsunomiya T, et al. Prognostic re-evaluation of peritoneal lavage cytology in Japanese patients with gastric carcinoma. Hepatogastroenterology. 2009; 56:261–265.34. Bando E, Yonemura Y, Taniguchi K, Fushida S, Fujimura T, Miwa K. Outcome of ratio of lymph node metastasis in gastric carcinoma. Ann Surg Oncol. 2002; 9:775–784.
Article35. Ryu CK, Park JI, Min JS, Jin SH, Park SH, Bang HY, et al. The clinical significance and detection of intraperitoneal micrometastases by ThinPrep(R) cytology with peritoneal lavage fluid in patients with advanced gastric cancer. J Korean Gastric Cancer Assoc. 2008; 8:189–197.
Article36. Nemes S, Jonasson JM, Genell A, Steineck G. Bias in odds ratios by logistic regression modelling and sample size. BMC Med Res Methodol. 2009; 9:56.
Article37. Sobin LH, Wittekind C, editors. TNM: classification of malignant tumours. 6th ed. New York: Wiley-Liss;2002.38. Wong JH, Johnson DS, Hemmings D, Hsu A, Imai T, Tominaga GT. Assessing the quality of colorectal cancer staging: documenting the process in improving the staging of node-negative colorectal cancer. Arch Surg. 2005; 140:881–886.39. Jha MK, Corbett WA, Wilson RG, Koreli A, Papagrigoriadis S. Variance of surgeons versus pathologists in staging of colorectal cancer. Minerva Chir. 2006; 61:385–391.40. Yoshikawa T, Sasako M, Sano T, Nashimoto A, Kurita A, Tsujinaka T, et al. Stage migration caused by D2 dissection with para-aortic lymphadenectomy for gastric cancer from the results of a prospective randomized controlled trial. Br J Surg. 2006; 93:1526–1529.
Article41. Ayantunde AA, Parsons SL. Pattern and prognostic factors in patients with malignant ascites: a retrospective study. Ann Oncol. 2007; 18:945–949.
Article42. Marutsuka T, Shimada S, Shiomori K, Hayashi N, Yagi Y, Yamane T, et al. Mechanisms of peritoneal metastasis after operation for non-serosa-invasive gastric carcinoma: an ultrarapid detection system for intraperitoneal free cancer cells and a prophylactic strategy for peritoneal metastasis. Clin Cancer Res. 2003; 9:678–685.43. Nanda K, McCrory DC, Myers ER, Bastian LA, Hasselblad V, Hickey JD, et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med. 2000; 132:810–819.
Article44. Burke EC, Karpeh MS Jr, Conlon KC, Brennan MF. Peritoneal lavage cytology in gastric cancer: an independent predictor of outcome. Ann Surg Oncol. 1998; 5:411–415.
Article45. Kodera Y, Nakanishi H, Ito S, Yamamura Y, Kanemitsu Y, Shimizu Y, et al. Quantitative detection of disseminated free cancer cells in peritoneal washes with real-time reverse transcriptase-polymerase chain reaction: a sensitive predictor of outcome for patients with gastric carcinoma. Ann Surg. 2002; 235:499–506.
Article46. Weiss L. Metastatic inefficiency. Adv Cancer Res. 1990; 54:159–211.
Article47. Yonemura Y, Kawamura T, Bandou E, Tsukiyama G, Endou Y, Miura M. The natural history of free cancer cells in the peritoneal cavity. Recent Results Cancer Res. 2007; 169:11–23.
Article48. Makino T, Fujiwara Y, Takiguchi S, Miyata H, Yamasaki M, Nakajima K, et al. The utility of pre-operative peritoneal lavage examination in serosa-invading gastric cancer patients. Surgery. 2010; 148:96–102.
Article49. Kodera Y, Yamamura Y, Shimizu Y, Torii A, Hirai T, Yasui K, et al. Peritoneal washing cytology: prognostic value of positive findings in patients with gastric carcinoma undergoing a potentially curative resection. J Surg Oncol. 1999; 72:60–64.
Article50. Bonenkamp JJ, Songun I, Hermans J, van de Velde CJ. Prognostic value of positive cytology findings from abdominal washings in patients with gastric cancer. Br J Surg. 1996; 83:672–674.
Article51. Majima T, Ichikura T, Mochizuki H. Prognostic significance of the cytologic features of free cancer cells in the peritoneal cavity of patients with gastric cancer. Surg Today. 2002; 32:35–39.
Article52. Miyashiro I, Takachi K, Doki Y, Ishikawa O, Ohigashi H, Murata K, et al. When is curative gastrectomy justified for gastric cancer with positive peritoneal lavage cytology but negative macroscopic peritoneal implant? World J Surg. 2005; 29:1131–1134.
Article53. Inoue M, Tsugane S. Epidemiology of gastric cancer in Japan. Postgrad Med J. 2005; 81:419–424.
Article54. Ahn HS, Lee HJ, Yoo MW, Jeong SH, Park DJ, Kim HH, et al. Changes in clinicopathological features and survival after gastrectomy for gastric cancer over a 20-year period. Br J Surg. 2011; 98:255–260.
Article55. Mulherin SA, Miller WC. Spectrum bias or spectrum effect? Subgroup variation in diagnostic test evaluation. Ann Intern Med. 2002; 137:598–602.
Article56. Strander B, Andersson-Ellström A, Milsom I, Rådberg T, Ryd W. Liquid-based cytology versus conventional Papanicolaou smear in an organized screening program: a prospective randomized study. Cancer. 2007; 111:285–291.
Article57. Siebers AG, Klinkhamer PJ, Grefte JM, Massuger LF, Vedder JE, Beijers-Broos A, et al. Comparison of liquid-based cytology with conventional cytology for detection of cervical cancer precursors: a randomized controlled trial. JAMA. 2009; 302:1757–1764.
Article58. Schiffman M, Solomon D. Screening and prevention methods for cervical cancer. JAMA. 2009; 302:1809–1810.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Detection of Intra-peritoneal Free Cancer Cell during Laparoscopic Staging of Patients with Advanced Gastric Carcinoma
- The Clinical Significance and Detection of Intraperitoneal Micrometastases by ThinPrep(R) Cytology with Peritoneal Lavage Fluid in Patients with Advanced Gastric Cancer
- Conventional Cytology Is Not Beneficial for Predicting Peritoneal Recurrence after Curative Surgery for Gastric Cancer: Results of a Prospective Clinical Study
- Significance of Carcinoembryonic Antigen Levels in Peritoneal Washings for Gastric Cancer Patients
- Role of Peritoneal Lavage Cytology and Prediction of Prognosis and Peritoneal Recurrence After Curative Surgery for Colorectal Cancer